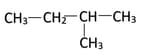
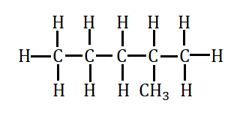
Isomerism
Isomerism: Overview
In this topic, we will deal with the concept of isomerism. We will study four types of structural isomerism, chain isomerism, position isomerism, functional group isomerism and metamerism in detail along with stereoisomerism.
Important Questions on Isomerism
What is the IUPAC name of the given structural isomer of hexane:
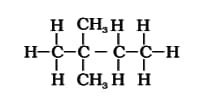
The number of structural isomers of hexane is:
Which one is the structural isomer of ?
Number of sigma bonds in neo-pentane is .
Which one is not the structural isomer of pentane?
Geometrical isomerism is possible with alkenes.
The first member of alkane that shows structural isomerism is:
For pentane total of three structural isomers are possible.
Identify the structural isomers of pentane.
Isomers are the chemical compound that has the same _____ formula but a different structural arrangement.
Which of the following statement is not true for the diastereoisomers?
A compound having three _____ would have the name propane.
Out of these which is the structural isomer of octane?
Which of the following pair of structures neither represent isomers nor different set of compounds?
has a chiral centre. Which one of the following represents its R-configuration ?