Fast and Slow Changes
Fast and Slow Changes: Overview
This topic covers concepts such as Preventive Measures of Rusting of Iron, Types of Changes around Us, Slow Changes, and Fast Changes.
Important Questions on Fast and Slow Changes
Choose the right word and fill in the blank provided below.
When we heat water in a pan, it begins to boil after some time. If we continue to heat further, the quantity of water in the pan will _____. (decrease, increase)
Give one word for the following:
Melting is the reverse of this process:
What happens to naphthalene balls kept in an almirah after a few days? Why?
Write the difference between physical and chemical changes with examples.
How many types of changes are there?
There is some reason behind every change.
Which of the following is a reversible change-
Observe the following pictures. Write S for a slow change and F for a fast change.
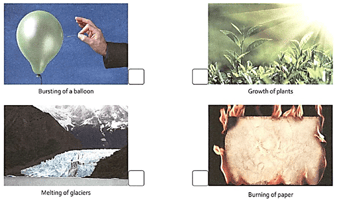
Give some examples of fast and slow changes that occur in your surroundings.
Changes that may take place on mixing two substances are always reversible.
Tell the difference between physical and chemical changes.
Describe how heat can cause substances to change.
Differentiate between physical and chemical changes with the help of examples.
List out common changes in surroundings.
Give two examples of changes which are exothermic.
The process of conversion of water into water vapour is called condensation.
Why are metal articles electroplated?
