Covalent Bond
Covalent Bond: Overview
This topic explains covalent bonds and its types. Covalent bonds in carbon and some examples of formation of covalent bonds such as formation of hydrogen molecules and formation of chlorine molecules are also explained here.
Important Questions on Covalent Bond
The number of electron pairs shared by the two carbon atoms which are bounded by a triple bond are:
Identify the different atom in the following compound.
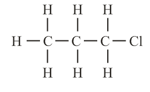
The number of coordinative covalent bonds in the structure of nitric acid is
Which one of the following compounds is not an ionic compound?
The high density of water compared to ice is due to
The number of covalent bonds in ethane are:
The number of covalent bonds present in pentane are
The interaction between a polar and non-polar molecule is known as
Which of the following bond is most polar covalent bond?
Which amongst the following has a polar covalent bonds?
The number of single and double bonds present in the structural formula of benzene is :
How many covalent bonds are present in isobutane ?
An unsaturated hydrocarbon having a triple covalent bond has hydrogen atoms in its molecule. The number of carbon atoms in its molecule will be:
Which of the following compounds is covalent?
