Special Types of Bonds and Non-bonded Forces of Attraction
Special Types of Bonds and Non-bonded Forces of Attraction: Overview
This topic covers concepts, such as Intermolecular Forces, London Dispersion Forces, Van der Waals Forces, Ion-dipole Forces, Dipole-dipole Forces, Dipole-induced Dipole Forces, Hydrogen Bonding & Applications of Hydrogen Bonding etc.
Important Questions on Special Types of Bonds and Non-bonded Forces of Attraction
Identify the nature of intermolecular force acting between and molecules.
Hydrogen bonding is found in:
Define hydrogen bonding.
An example of dipole-dipole force is:
_____ are weakest among van der Waals forces.
Define van der waals force.
London forces are long distance forces.
Dispersion forces are also known as _____.
Define intermolecular forces among particles.
What are dipole-induced dipole forces?
Three types of potential energy due to attractive interaction between two species and are represented by the curves and in the figure below.
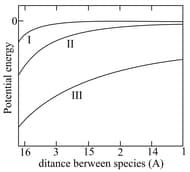
Consider the dominating interaction between non-rotating species for , and . The correct assignment of the ... interactions
to the types , and is:
Explain the type of bonding in water ?
Describe the dipole-induce dipole forces.
Hydrogen bond is a special case of
van der Waal's constant ' ' has the dimensions of
Identify a pair of molecules which shows dipole-induced dipole forces.
Explain briefly the dipole-induced dipole forces among the particles?
Which of the following bonds can be present in a chemical compound?
There are two types of hydrogen bonding.
Which of the following show intramolecular hydrogen bonding?
