Types of Chemical Reactions
Types of Chemical Reactions: Overview
This topic covers concepts, such as, Types of Chemical Reactions, Combination Reactions, Oxidation Reactions & Reduction Reactions etc.
Important Questions on Types of Chemical Reactions
The following questions consist of two statements- Assertion(A) and Reason(R). Answer these questions selecting the appropriate option given below:
Assertion(A) : In electrolysis of water, the volume of hydrogen liberated is twice the volume of oxygen formed.
Reason (R) : Water, has hydrogen and oxygen in the ratio of by volume.
Assertion: In exothermic welding, aluminium dust reduces the oxide of another metal, most commonly iron oxide.
Reason: Aluminium is highly reactive.
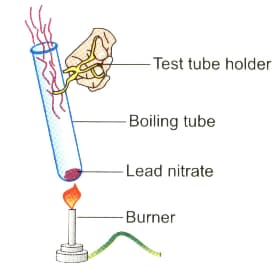
Observe the diagram given below and answer the following questions:
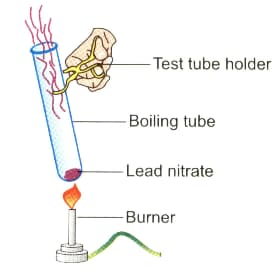
Observe the diagram given below and answer the following questions:
Two gases are evolved during this reaction, one is nitrogen dioxide. Which is the other?
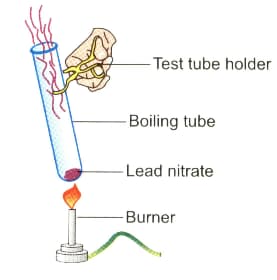
Observe the diagram given below and answer the following questions:
A precipitate is formed in this reaction, what colour is the precipitate?
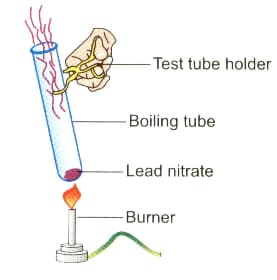
Observe the diagram given below and answer the following questions:
What type of reaction is this?
The above chemical is called _____ reaction?
Why the reaction between lead and copper sulphide is displacement?
In single _____ reaction, lead has displaced or removed another element, copper from copper sulphide solution.
In which of the following reaction, Lead has displaced or removed another element, copper, from copper chloride solution.
The burning of natural gas is oxidation as well as an endothermic reaction.
Complete the following equation:
_____ .
(Enter your correct answer as )
Write a balanced chemical equation for the following reaction:
Burning of natural gas.
Identify the type of reaction for the following reaction:
Burning of natural gas.
Identify the type of reaction for the following reaction:
is a redox reaction.
What is the type of reaction that occurs when reacts with carbon?
Identify the reducing and the oxidising agents for the following reaction:
Which of the following is not an exothermic change?
Explain what is meant by a highly exothermic” reaction and explain why such a reaction needs to be carried out in a safe and controlled way.
