Indicators
Indicators: Overview
This topic provides information about indicators and explains how they change colours according to the pH alteration. We will learn in detail the behaviour of indicators. It also briefs on the properties of indicators and characteristics of a good indicator.
Important Questions on Indicators
A solution turns methyl orange into yellow the approximate of solution is
What happens, when methyl orange solution mixed with ?
The indicators which turn red in acid solution are:
Which of the following is not an acid-base indicator?
Methyl orange shows _____ colour in acidic medium and yellow colour in basic medium.
The colour of phenolphthalein indicator in basic solution is:
What colour does the litmus solution indicator turn when added to a base?
On burning a piece of charcoal, a gas evolves. How is it possible to know whether the gas is acidic or basic in nature.
Which of the following is not a naturally occuring indicator?
Methyl orange changes its colour to yellow when added to a base or an alkali. (True/False)
Select the correct statements regarding solutions given in test tubes and .
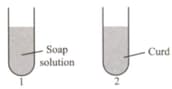
I. Solution will turn methyl orange indicator red.
II. Solution will turn turmeric indicator red.
III. Solution will turn methyl orange indicator yellow.
IV. Solution will turn China rose indicator magenta.
At range of phenolphthalein is . At range of phenolphthalein would be-
The rapid change of pH near the stoichiometric point of an acid-base titration is the basis of indicator detection. pH of the solution is related to ratio of the concentrations of the conjugate acid (Hln) and base (ln-) forms of the indicator by the expression
