Types of Chemical Reactions
Types of Chemical Reactions: Overview
This topic covers concepts such as Types of Chemical Reactions, Combination Reactions, Reaction between Calcium Oxide and Water, Reaction between Calcium Hydroxide and Carbon Dioxide, Applications of Calcium Hydroxide, etc.
Important Questions on Types of Chemical Reactions
Assertion:(A) A white washed wall develops a coating of calcium carbonate after a few days.
Reason:(R) Calcium oxide on the wall reacts slowly with carbon dioxide in the air.
The following questions consist of two statements- Assertion(A) and Reason(R). Answer these questions selecting the appropriate option given below:
Assertion(A) : In electrolysis of water, the volume of hydrogen liberated is twice the volume of oxygen formed.
Reason (R) : Water, has hydrogen and oxygen in the ratio of by volume.
Observe the diagram given below and answer the following questions:
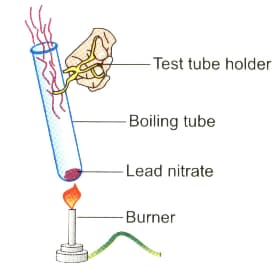
What type of reaction is this?
The above chemical is called _____ reaction?
Why the reaction between lead and copper sulphide is displacement?
In single _____ reaction, lead has displaced or removed another element, copper from copper sulphide solution.
In which of the following reaction, Lead has displaced or removed another element, copper, from copper chloride solution.
The burning of natural gas is oxidation as well as an endothermic reaction.
Complete the following equation:
_____ .
(Enter your correct answer as )
Write a balanced chemical equation for the following reaction:
Burning of natural gas.
Identify the type of reaction for the following reaction:
Burning of natural gas.
Identify the type of reaction for the following reaction:
is a redox reaction.
What is the type of reaction that occurs when reacts with carbon?
Identify the reducing and the oxidising agents for the following reaction:
Which of the following is not an exothermic change?
Explain what is meant by a highly exothermic” reaction and explain why such a reaction needs to be carried out in a safe and controlled way.
A student proposes to make some potassium nitrate. She uses the following method. First, she reacts a small amount of potassium metal with water. This creates an alkaline solution of potassium hydroxide. She then adds a small amount of dilute nitric acid to create potassium nitrate.
Give two safety precautions that she should take when carrying out this method.
of each of the elements with atomic numbers to was burned in chlorine gas. In each case there was an exothermic reaction and the amount of energy released was measured. The results are shown in the table.
| Atomic number | Energy released |
No measurement was made for the element with atomic number . Use your graph to predict the amount of energy released.
of each of the elements with atomic numbers to was burned in chlorine gas. In each case there was an exothermic reaction and the amount of energy released was measured. The results are shown in the table.
| Atomic number | Energy released |
The element with atomic number did not react with chlorine gas. Suggest why is it so?
