Separation of Components of a Mixture
Separation of Components of a Mixture: Overview
In this topic, we will study separation methods of two components in a matter. It highlights evaporation, filtration, and separation of immiscible liquids using separating funnels, sublimation, chromatography, distillation, and fractional distillation.
Important Questions on Separation of Components of a Mixture
Define the principle of chromatography.
The mixture of salt and camphor can be separated by the _____ method.
Which separation technique is used to separate the components from petroleum?
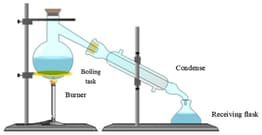
Label the figure given below.
Where and why is it used?
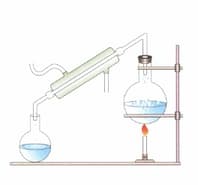
Label the figure given below.
What is the process called?
Chromatography is used to separate mixture of chemicals in their liquid or gaseous form.
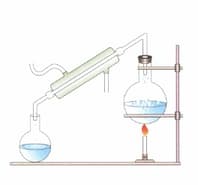
Observe the image. Explain the method and why is it used?

Differentiate between: Distillation and Fractional distillation.
State whether the following statements are True or False.
The process to obtain pure substance from a mixture in a solution is called distillation.
What is the principle used in distillation?
On what principle does chromatography work?
Observe the diagram carefully. Point out the place where water vapour condenses into water.
A liquid with boiling point189$ ℃$ is soluble in water.

Which of the following processes can be used to separate a colourless solution of the liquid from water?
State true or false.
The picture shows the formation of water vapour inside the condenser during the distillation of salt solution. These water vapours are free from any dissolved solid impurities.
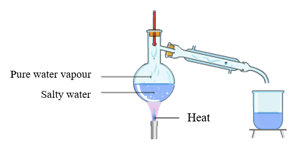
In a severe water contamination in home water supply which of the following is the recommended method for removal of soluble impurities from water?
Ink is soluble in water. How can you get pure water from a mixture of ink and water?
A mixture of oil and water can be separated using _____.
(Chromatograph, Valency, Cuprum, Negatively, , one, , Positively, Separating funnel)
A mixture of ethanol and water can be separated by using which of the following methods?
Which mixture can be separated using distillation?
During the process of sublimation, a liquid is converted into its vapours on heating.