Metallurgy of Iron
Metallurgy of Iron: Overview
This topic covers concepts such as Blast Furnace, Extraction of Iron, Slag Formation, Flux, Pig iron, Wrought iron and Iron Ore.
Important Questions on Metallurgy of Iron
During the extraction of haematite, limestone is added which acts as _____.(flux/slag)
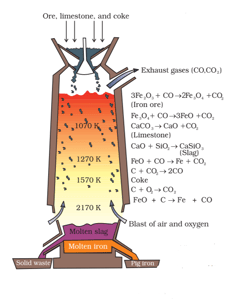
The above process diagram of a machinery that is used in the extraction of iron. The diagram is of a _____.
A substance is formed when of carbon is added to wrought iron.
Answer the question: A type of iron which has carbon content.
Write the three forms of iron and give reason, why they are different from each other?
During smelting an additional substance is added which combines with impurities to form a fusible product. Name the substance.
In extraction of iron, limestone is used for:
Write short notes on the following-
slag
Write short notes on the following-
flux
Suggest a flux for the removal of silica gangue.
Which is the effective reducing agent in the extraction of iron from haematite?
is reduced to spongy iron near the top of blast furnace by:
In metallurgy, flux is a substance used to convert:
In blast furnace, iron oxide is reduced by:
Write about any two different furnaces used for extraction of metals?
Write reactions involved at different temperatures in the blast furnace.
Which is the purest form of iron.