Metals
IMPORTANT
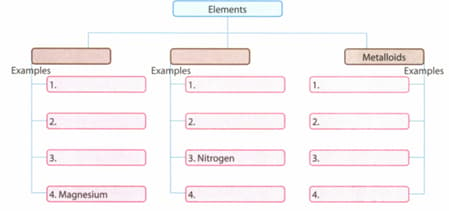
Metals: Overview
This topic covers concepts such as cation, hardness, electronic configuration of metals, metals and non-metals, and melting point of metals.
Important Questions on Metals
MEDIUM
IMPORTANT
Name two metals that have low boiling points.
MEDIUM
IMPORTANT
_____ are three metals that have densities lower than water.
EASY
IMPORTANT
_____ is the positively charged atom or group of atoms.
MEDIUM
IMPORTANT
Explain why the size of the cation is smaller than its corresponding atom.
MEDIUM
IMPORTANT
Name the products formed when sulphur reacts with the concentrated nitric acid.
MEDIUM
IMPORTANT
Why are metals good conductors of electricity?
EASY
IMPORTANT
All the metals are non-conductors of electricity.
HARD
IMPORTANT
How can you examine that metals conduct electricity? Explain with the help of an activity?
HARD
IMPORTANT
Give a comparative account between metals and non-metals with examples.