Gay-Lussac's Law
Gay-Lussac's Law: Overview
In this topic, we will study the Gay-Lussac's law of gaseous volumes. This law tells us that pressure and temperature remain constant when gases react with each other. We will also explore some applications of this law.
Important Questions on Gay-Lussac's Law
In the Haber process of dihydrogen and of dinitrogen were taken for reaction which yielded only of the expected product. What will be the composition of gaseous mixture under the aforesaid condition in the end?
In Haber's process, of dihydrogen and of dinitrogen were taken for the reaction, which yielded only of the expected product. What will be the composition of the gaseous mixture under the aforesaid condition in the end?
hydrogen is reacted with oxygen to give of water. The value of is
What is the maximum mass of that can be precipitated by mixing of with ot
| Molar mass | |
| 461 |
1.
2.
3.
Blister copper is produced by reaction of copper oxide with copper sulphide.
When of and of are used for reaction, the mass of copper produced is .
(Atomic mass of )
Consider the reaction
If the reaction started with of and of , then calculate the number of moles of formed.
A container of an ideal gas that is isolated from its surroundings is divided into two parts. One part has double the volume of the other. The pressure in each part is and the temperature is the same. The partition is removed. What is the pressure in the container now?
When litres of is mixed with litres of each at STP, the moles of formed is equal to
The expansion of an ideal gas of mass in volume at constant pressure is given by the straight line. Then the expansion of the same ideal gas of mass at a pressure is given by the straight line
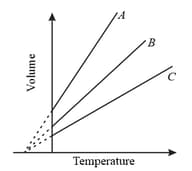
Air is filled in a bottle at atmospheric pressure and it is corked at . If the cork can come out at a pressure times the atmospheric pressure, then up to what temperature should the bottle be heated in order to remove the cork?
When gases react together, their reaction volume bears a simple ratio to each other, under the same conditions of temperature and pressure. This law was proposed by _____.
What is the maximum mass (in grams) of that could be obtained from of and of when they react? The balanced equation is
Equal weights of (atomic weight) and (atomic weight) react to form the compound, . If that is the case, then
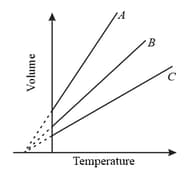
In the figure, the straight line shows the expansion of an ideal gas of volume and mass at a constant pressure . Which straight line shows the expansion for the same ideal gas if mass and pressure are doubled.
Find the limiting reagent in the given reaction, if any in the following reaction mixtures. The given reaction is
(i) 300 atoms of A + 200 molecules of B
(ii) 2 moles of A + 3 moles of B
(iii)100 atoms of A + 100 molecules of B
(iv) 5 moles of A + 2.5 moles of B
(v) 2.5 moles A + 5 moles of B
The maximum number of moles of that can be formed if is mixed with is
If of is treated with of . Which of the reactants will act as limiting reagent?
Calculate the amount of which is left unreacted in the given reaction:
If of is mixed with of .
How many moles of precipitate product formed when 72 moles of react with moles of ?
moles of and moles of are allowed to react completely according to reaction
The number of moles of formed is :
