Atomic Number and Atomic Weight
Atomic Number and Atomic Weight: Overview
This topic explains concepts such as, Atomic Numbers, Mass Numbers, Nucleons, etc.
Important Questions on Atomic Number and Atomic Weight
The mass of positively charged particles present in an atom is found to be times that of an electron. Identify the element and write its electronic configuration.
Which of the following options shows the correct set of atomic numbers and valency for element X in a neutral state as given below?
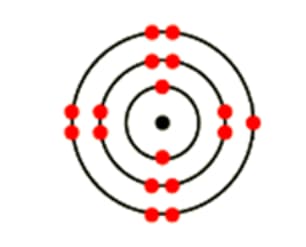
Explain with example: Mass number
A metal (mass number ) having same number of protons and neutrons, combines with two chlorine atoms. Identify the element with which electronic configuration of this metal matches in combined state.
What is the atomic number of iron?
What is the atomic number of helium?
Find the number of electrons, protons and neutrons in .
Find the number of protons and neutrons in the nucleus of an atom of an element which is represented as .
Two elements P and Q have and electrons in their outermost shell respectively. The atomic number of P and Q will be
Which of the following statement is always correct?
The number of electrons in an element X is 14 and the number of neutrons is 16.Which one of the following is the correct representation of the element?
If you know the mass number and the atomic number of an atom, how might you calculate its number of neutrons?
Write the definition of mass number.
Write the definition of atomic number.
The nucleus of an atom has its mass.
What is a periodic table?
Protons and neutrons are collectively called as;
Elements arranged in each group of periodic table have the same number of valence electrons.
There are total 113 elements known so far in the periodic table.
Total number of protons and neutrons in atoms of elements is called periodic table.
