Valency
Valency: Overview
This Topic covers sub-topics such as Valency, Octet Rule, Valency of the First 18 Elements, Valencies of Noble Gases and, Calculation of Valency of Elements
Important Questions on Valency
The ratio of non-metal atoms to the number of atoms of two non-metallic oxides A and B are and , respectively. Find the valencies of non-metals present in those non-metallic oxides.
What is the general name of the elements having electrons in the valence shell of their atoms?
Choose from the following list, as to what matches the descriptions below:
A cation whose electronic configuration resembles argon.
Enter your answer as or
An outermost-shell, which had eight electrons was said to possess _____ .
The electrons present in the outermost shell of an atom are known _____ .
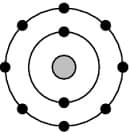
The following diagram represents the atomic structure of which element?
An element X has valency equal to 3. What will be its formula with carbonate ions?
An element has an electronic configuration of 2, 8, 7. Its valency is
The element X has 2 valence electrons. It is a _____ .
Three elements A, B and C have atomic number Z-1, Z and Z + 2 respectively. B is a noble gas. The compound between A and C will be :
Which of the following will have equal number of electrons ?
Which of the following is correct electronic configuration of argon ?
If the electronic configuration of elements 'A' and 'B' are respectively, then the formula of the compound formed by the combination of these elements will be-
