How Strong are Acid or Base Solutions?
Important Questions on How Strong are Acid or Base Solutions?
What are strong and weak acids? In the following list of acids, separate strong acids from weak acids. Hydrochloric acid, citric acid, acetic acid, nitric acid, formic acid, sulphuric acid.
A student prepared solution of (i) an acid and (ii) a base in two separate beakers. She forgot to label the solution and litmus paper is not available in the laboratory. Since, both the solutions are colourless, how will she distinguish between the two?
Name the acid present in ant sting and give its chemical formula. Also, give the common method to get relief from the discomfort caused by the ant sting.
What will be the action of the following substance on litmus paper? Dry gas, moistened gas, lemon juice, Carbonated soft drink, curd, soap solution.
Which of the following is used for dissolution of gold?
In an attempt to demonstrate conductivity through an electrolyte, the following apparatus (figure) was set up.
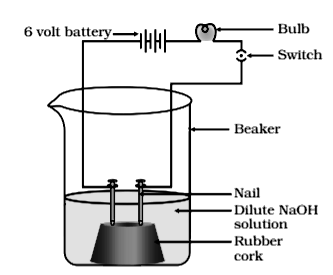
Which among the following statement(s) is/are correct?
(i) Bulb will not glow because the electrolyte is not acidic.
(ii) Bulb will glow because is strong base and furnishes ions for conduction.
(iii) Bulb will not glow because the circuit is incomplete.
(iv) Bulb will not glow because it depends upon the type of electrolytic solution.
Which of the following is acidic in nature?
Which one of the following can be used as an acid-base indicator by a visually impaired student?
The pH of the gastric juices released during digestion is
To protect tooth decay we are advised to brush our teeth regularly. The nature of the toothpaste commonly used is
One of the constituents of baking powder is sodium hydrogen carbonate, the other constituent is
Common salt besides being used in kitchen can also be used as the raw material for making
(i) washing soda
(ii) bleaching powder
(iii) baking soda
(iv) slaked lime
A sample of soil is mixed with water and allowed to settle. The clear supernatant solution turns the pH paper yellowish-orange. Which of the following would change the colour of this pH paper to greenish-blue?

