Types of Chemical Reactions
Important Questions on Types of Chemical Reactions
Which of the following is an example of a combination reaction?
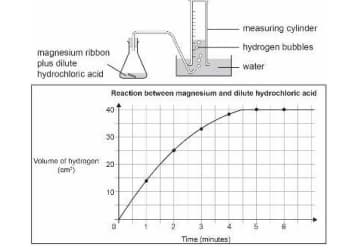
A piece of magnesium ribbon is added to a flask containing dilute hydrochloric acid.
Hydrogen gas is formed which is collected in the measuring cylinder.
The line on the graph indicates the rate of chemical reaction occurring in the flask.
The amount of hydrogen formed with time is plotted on a graph.
 .
.
Which of these could increase the rate of reaction in the flask?
The equation given below shows the reaction for cellular respiration. Cellular respiration is a chemical process by which cells convert glucose to energy.
Carbon dioxide and water are the two new substances formed during cellular respiration. What are they known as?
Methane gas released from waste water treatment plants can be used as a source of fuel. Which chemical equation represents combustion of methane to produce heat energy?
Which of these is an example of decomposition reaction?
Magnesium reacts with hydrochloric acid to form magnesium chloride and hydrogen gas.
Write a balanced chemical equation to show the reaction.
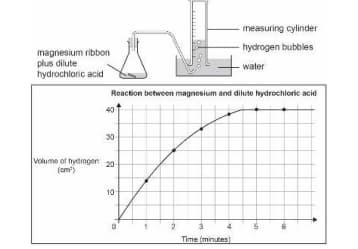
A piece of magnesium ribbon is added to a flask containing dilute hydrochloric acid.
Hydrogen gas is formed which is collected in the measuring cylinder.
The line on the graph indicates the rate of chemical reaction occurring in the flask.
The amount of hydrogen formed with time is plotted on a graph.
 .
.
The reaction is repeated with magnesium powder in place of magnesium ribbon under the same conditions. Will the reaction rate increase or decrease?
Explain your answer with reference to the volume of hydrogen formed in the flask at 2 minutes.
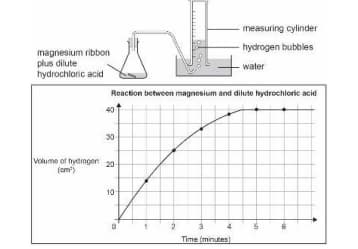
A piece of magnesium ribbon is added to a flask containing dilute hydrochloric acid.
Hydrogen gas is formed which is collected in the measuring cylinder.
The line on the graph indicates the rate of chemical reaction occurring in the flask.
The amount of hydrogen formed with time is plotted on a graph.
 .
.
At what time is the reaction rate the fastest in the flask?
Tina finds a paper covered with a white substance in a chemistry lab.She keeps the paper near the window of the lab and comes back to pick it up after five hours to take it home. She noticed that the white substance had turned grey.
State one application of this property of the substance seen in daily life.
Tina finds a paper covered with a white substance in a chemistry lab. She keeps the paper near the window of the lab and comes back to pick it up after five hours to take it home. She noticed that the white substance had turned grey.
The substance changed from white to grey. Write the chemical equation for this reaction.
Tina finds a paper covered with a white substance in a chemistry lab. She keeps the paper near the window of the lab and comes back to pick it up after five hours to take it home. She noticed that the white substance had turned grey.
What could be the most likely substance on the paper that Tina found?
Write the balanced chemical equation of any one reaction that CANNOT be classified as combination, decomposition, simple displacement or double displacement.
Dilip was comparing combination reactions with decomposition reactions.
Which class of chemical substances may be the product of a decomposition reaction but NOT a product of a combination reaction?
Trupti mixes an aqueous solution of sodium sulphate and an aqueous solution of copper chloride
Will this lead to a double displacement reaction? justify your answer.
The diagram below shows the set-up in which the electrolysis of water takes place.
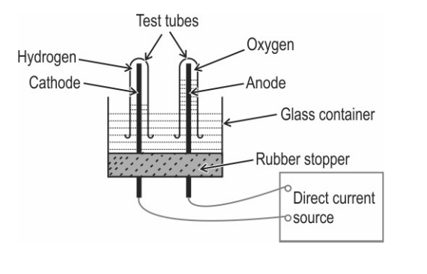
The test tube containing hydrogen is removed carefully from the apparatus. A lit match stick is brought near the mouth of this test tube. The gas burns with an explosive "pop" sound.
Write a balanced chemical equation for the above electrolysis reaction and indicate whether energy is absorbed or released.
The diagram below shows the set-up in which the electrolysis of water takes place.
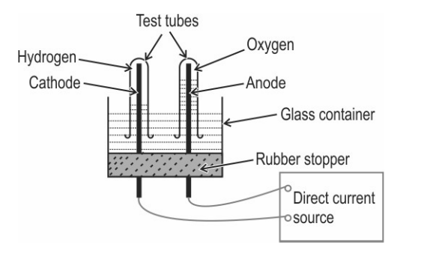
Explain why this is an example of an endothermic reaction?
The diagram below shows the set-up in which the electrolysis of water takes place.
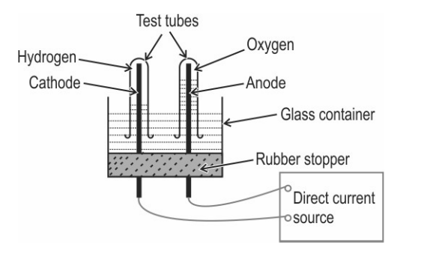
What type of reaction takes place?
Which of the following is an example of simple displacement reaction?
The change in colour of moist litmus paper in the given set up is due to :
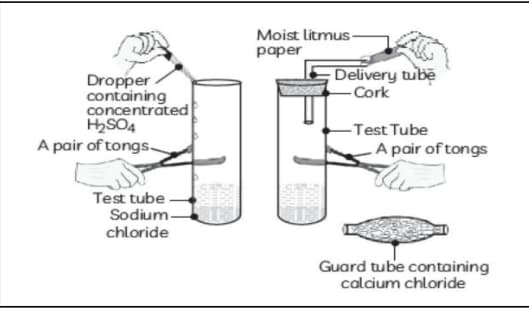
i. Presence of acid
ii. Presence of base
iii. Presence of (aq) in the solution
iv. Presence of litmus which acts as an indicator.
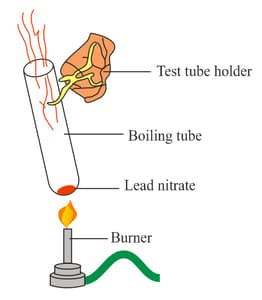
Rahul is a very curious student who frequently visits the lab in his free time. Rahul was interested in conducting an experiment, and he let the teacher know about the same. The teacher gave him a test tube containing lead nitrate and instructed him to heat it up and watch what happened. He began heating the test tube, and after a while, brown-coloured vapours were coming out. The teacher then asked Rahul to analyse the reaction taking place. Could you assist Rahul in identifying the type of the reaction and its chemical equation? Also, help Rahul to identify the gas evolved as brown vapours.