Latent Heat and Specific Latent Heat
Latent Heat and Specific Latent Heat: Overview
This topic gives us a detail on the latent heat of fusion and vaporisation along with the specific latent heat. It also discusses the factors affecting the melting point and the boiling point with the activities.
Important Questions on Latent Heat and Specific Latent Heat
How much heat is required to convert of ice at to water at ? (Given specific heat of ice , specific heat of water , Latent heat of fusion )
of steam at is passed into a large block of ice at the mass of ice that melts is
What is the total heat energy present in one gram of water?
Water and ice having equal masses are placed inside an insulated container. The initial temperatures of water and ice are and respectively. The equilibrium temperature of the system in degrees Celsius is close to (Note: take the specific heat of water ; latent heat of fusion )
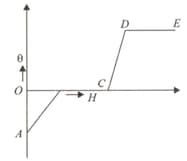
Heat given (H) to a substance was plotted against rise in temperature (0). Which of the following parts of the graph, most correctly
depicts the latent heat of the substance?
An electric heater raises the tempeature of of a given liquid from to in . If the power of the heater is , calculate:
(a) the heat capacity of the liquid.
(b) the specific heat capacity of the liquid.
of ice at is added to of a liquid at . What will be the final temperature of the mixture when all the ice gets melted? The specific heat capacity of the liquid is , while that of water is . (Take, specific latent heat of fusion of ice = )
State the principle of method of mixture and what other name is given to it?
Explain the electric method to calculate the specific latent heat of ice?
The specific latent heat of vaporization of the water is .
Will the value of specific latent heat of a substance change if the scale is instead of ?
The S.I. unit of specific latent heat is _____ .
Explain the term ‘specific latent heat of fusion’ of a substance.
What is specific latent heat of vaporization of a substance?
What are the effects of Latent Heat of Fusion of Ice in Daily Life?
Give two consequences of high latent heat of steam.
Explain why if the ice at the top of the mountain never melts together completely.
Heat is supplied to a solid to raise its temperature across its melting point and boiling point Which of the following graphs correctly represents the relation between heat supplied and temperature ?
The specific latent heat of vaporisation for water is large.
The correct reason behind cooling of a room after the water has been sprinkled is