Calorimetry
Calorimetry: Overview
This Topic covers sub-topics such as Calorimetry, Calorimeter, Specific Heat Capacity and, Heat Capacity
Important Questions on Calorimetry
A body of mass falls from a height of and rebounds to a height of . If the loss in energy goes into heating the body, then the rise in temperature will be nearly: (specific heat of material is )
Below is a graph between change in internal energy and temperature for a system placed in a constant volume.
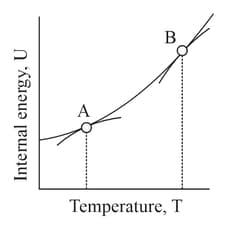
The slope of the graph is known as
Which one of the following material will expand maximum if the same amount of heat energy is given to them?
of energy supplied to of water will raise its temperature by nearly
Two beakers and contain liquids of mases and respectively and specific heats and . The amount of heat on them is equal. If they are joined by a metal rod
Three identical silver cups and contain three liquids of same densities at same temperature higher than the temperature of the surrounding. If the ratio of their specific heat capacities is , then
If the heat given to raise the temperature of two solid spheres of radius and and density and through is same, then the ratio of their specific heat capacity is:
In a certain system of units, the fundamental unit of mass is taken as unit of length is taken as unit of time taken as and unit of temperature is taken as Kelvin. In this system. unit of specific heat capacity will be (specific heat capacity is given by Where is heat, is mass and is change in temperature) :-
Why the container of calorimeter is made up of copper?
The SI unit of specific heat capacity is joule per kelvin per kilogram.
The SI unit for heat capacity is joule per kelvin.
If calories of heat are supplied to of water, its temperature rises from to If specific heat for water is , then the value ofis
Equal masses of three liquids have temperatures respectively. If are mixed, the mixture has a temperature of . If are mixed, the mixture has a temperature of . If are mixed, then the mixture will have a temperature in
Two different liquids of same mass are kept in two identical vessel, which are placed in a freezer that extracts heat from them at the same rate causing each liquid to transform into a solid. The schematic figure below shows the temperaure vs time plot for the two materials. We denote the specific heat in the liquid states to be and for materials and respectively, and latent heats of fusion and respectively.
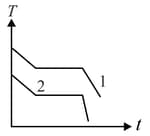
Choose the correct option.
Ice melts when energy in the form of ____ is supplied
200 g water is heated from to . Ignoring the slight expansion of water, the change in its internal energy is close to (Given specific heat of water J/kg/K):
A current carrying wire heats a metal rod. The wire provides a constant power to the rod. The metal rod is enclosed in an insulated container. It is observed that the temperature in the metal rod changes with time as:
where is a constant with appropriate dimension while is a constant with dimension of temperature. The heat capacity of the metal is:
