Movement in a Redox Reaction
Movement in a Redox Reaction: Overview
This Topic covers sub-topics such as Reactivity Series of Metals, Displacement Reactions, Reducing Agents, Oxidising Agents, Redox Reactions in Terms of Electron Transfer Reactions and, Reaction between Iron and Copper Sulphate
Important Questions on Movement in a Redox Reaction
Which metal is displaced when lead is put in the solution of copper chloride?
has a structure as shown below:
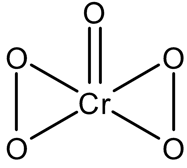
The oxidation number of chromium in the above compound is
Which of the following pairs will give displacement reactions?
If and represent metals in the decreasing order of their reactivity, which one of them is most likely to occur in a free state in nature?
When ethanol is heated with alkaline potassium permanganate solution, it gets converted into ethanoic acid. In this reaction, alkaline potassium permanganate acts as:
Calcium is a less reactive metal than sodium.
Calcium is more reactive metal than potassium.
Respiration can be classified as which of the following processes?
The reaction in which hydrogen is gained is called:
Both alkali metals and hydrogen act as_____.
A more reactive substance displaces a less reactive substance in a displacement reaction.
On the basis of the sequence of the given reactions identify the most and least reactive elements:
...(i)
...(ii)
...(iii)
