Titration
Titration: Overview
This Topic covers sub-topics such as Titration, Titration of a Strong Acid versus a Weak Base, Titration of a Strong Acid versus a Strong Base, Titration of a Weak Acid versus Strong Base and, Titration of a Weak Acid versus a Weak Base
Important Questions on Titration
Which is/are correct statements?
(i) In any strong acid solution, the concentration of will be zero.
(ii) If of a reaction is positive, then the reaction will not proceed at all, in the forward direction for any concentrations of reactants and products.
(iii) Titration curves are drawn for (about the figure shown).
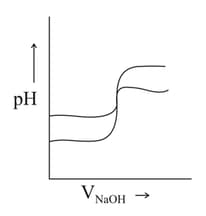
(a) with and(b) with on the same graph paper they look like
What is the volume of hydrogen chloride(HCl) required for complete reaction of sodium bicarbonate(NaHCO3) in a double titration method, for the mixture of sodium bicarbonate(NaHCO3) and sodium carbonate (Na2CO3).If the volume of hydrogen chloride(HCl) used at phenolphthalein indicator end point was x ml and further of Methyl orange indicator end point was y ml.
