Bond Parameters
Bond Parameters: Overview
This Topic covers sub-topics such as Bond Order, Bond Length, Bond Enthalpy, Bond Angle, Bond Parameters, Bond Enthalpy for Molecules containing Multiple Bonds, Factors Affecting Bond Angles of Molecules and, Mean or Average Bond Enthalpy
Important Questions on Bond Parameters
For phenol, which of the following resonating structure is the most stable?
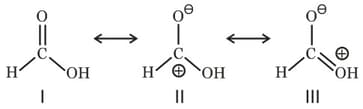
Among these canonical structures, the correct order of stability is
Bond formation is :
Which of the following statements are true.
(i) In the structure of the bond is shorter than the bond
(ii) All the bonds in are not equivalent.
(iii) is more reactive than
In the compound,  , the bond is of the type –
, the bond is of the type –
Among the following, the molecule with the highest dipole moment is
Which of the following compounds, hydrocarbons, has the lowest dipole moment?
A metal, M forms chlorides in its and Oxidation states. Which of the following statements about these chlorides is correct?
Among the following chloro-compound having the lowest dipole moment is:
The number of following factors which affect the percent covalent character of the ionic bond is______
A) Polarising power of cation
B) Extent of distortion of anion
C) Polarisability of the anion
D) Polarising power of anion
The bond order and magnetic property of acetylide ion are same as that of
Consider the following statement
(A) molecules has a trigonal planar structure.
(B) Bond Length of is shorter than .
(C) Isoelectronic molecules or ions have identical bond order.
(D) Dipole moment of is higher than that of water molecule.
Choose the correct answer from the options given below:
Which one of the following pairs is an example of polar molecular solids?
The pair from the following pairs having both compounds with net non-zero dipole moment is
The number of following factors which affect the percent covalent character of the ionic bond is______
How many factors will contribute to major role in covalent character of a compound?
a. Polarising power of cation
b. Polarisability of the anion
c. Distortion caused by cation
d. Polarisability of cation
What is Fajan rule?
Write resonance structure of .
What is dipolarisation?
Assuming the bond direction to be axis, which of the overlapping of atomic orbitals of two atoms will be result in bonding:
s orbital of and orbital of
s orbital of and orbital of
orbital of and orbital of
s- orbitals of
