Resonance
Resonance: Overview
This topic covers concepts such as conditions for resonance, resonance, resonance energy or canonical energy, and resonating structures of compounds.
Important Questions on Resonance
For phenol, which of the following resonating structure is the most stable?
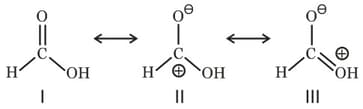
Among these canonical structures, the correct order of stability is
In which of the following compounds resonance does not occurs :
(a) (b) (c) (d)
The bond in is shorter than bond in Account for this observation.
What is the need of resonating structure for a molecule?
Why do we need to draw resonating structures for a molecule?
Discuss the necessary condition for resonance.
Assertion. The resonance hybrid is more stable than any of the contributing structure.
Reason. The contributing structures contain the same number of unpaired electrons and have real existence.
Consider the following resonating structure. What will be the correct decreasing order of stability?
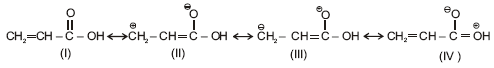
In carbonate ion, all three carbon - oxygen bonds are equivalent in all respects, viz, length, energy. The average bond order of the carbon-oxygen bond is
Round of your answer up to two decimal places.
Which of the following statements are correct for butadiene
(1) The and bonds are longer than a carbon-carbon double bond.
(2) The and bonds are shorter than a carbon-carbon double bond.
(3) The bond is slightly shorter than a carbon-carbon single bond.
(4) The bond is slightly longer than a carbon-carbon single bond.
Select the incorrect statement about the following
Which of the following molecules does not show any resonating structures?
The nitrite ion in represented by
(I)
(II)
Which of the structures represents possible resonance forms of this ion?
