Chemical Properties of Carboxylic Acids
Chemical Properties of Carboxylic Acids: Overview
This topic consists of various concepts like Reactions of Carboxylic Acids involving Cleavage of C-O Bond,Reactions of Carboxylic Acids involving Cleavage of O-H Bond,Mechanism of Esterification, etc.
Important Questions on Chemical Properties of Carboxylic Acids
The correct increasing order of acidic strength of the following compounds is
benzoic acid
nitro benzoic acid
, dinitrobenzoic acid
methoxybenzoic acid
Identify A to D in the following sequences of operations:
The reagents that can be used to distinguish between the following pairs of compounds would be:
(i) Propanal and Propanone
(ii) Phenol and Benzoic acid
Acetyl bromide reacts with excess of followed by treatment with a saturated solution of gives –
Among the following acids which has the lowest value?
A liquid was mixed with ethanol and a drop of concentrated H2SO4 was added. A compound with a fruity smell was formed. The liquid was:
A liquid was mixed with ethanol and a drop of concentrated H2SO4 was added. A compound with a fruity smell was formed. The liquid was:
Which amongst the following compounds has the highest acid strength?
An organic compound with molecular formula , which is a colourless liquid at room temperature with unpleasant odour, onnreaction with Phosphorous trichloride gives compound . Compound on treatment with dimethyl cadmium gives compound , which when treated with sodium hypoiodite gives yellow colour precipitation. Identify compounds and write the sequence of the chemical reactions involved.
Given below are two statements, one is labelled as Assertion and the other is labelled as Reason .
Assertion : A solution of the product obtained by heating a mole of glycine with a mole of chlorine in presence of red phosphorous generates chiral carbon atom.
Reason : A molecule with chiral carbons is always optically active.
In the light of above statements, chose the correct answer from the options given below:
Complete the following reaction :
Which acid of the following pair is a stronger acid ?
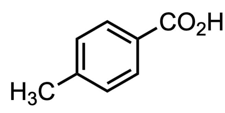 or
or 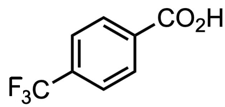
Match List-I with .
|
List-I Name of reaction |
List-II Reagent used |
||
| Hell-Volhard Zelinsky reaction | |||
| Iodoform reaction | |||
| Etard reaction | |||
| Gatterman-Koch reaction | |||
Choose the correct answer from the options given below:
The major product formed in the following reaction is
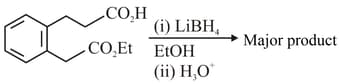
Compare the acid strength of:
Find the correct order of acidity of the following compounds :
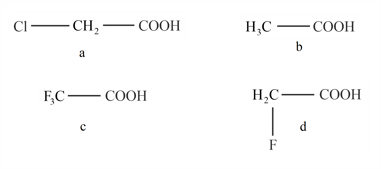
Match the following
| Name of the Reaction | Reagents |
| (a) Etard reaction | (p) |
| (b) Iodoform | (q) |
| (c) Gattermann | (r) |
| (d) HVZ | (s) |
Identify the products.
Which of the following acids has least acidic strength ?
Convert the following :
Ethanol to propanol
