Nomenclature and Structure of Carbonyl Group
Nomenclature and Structure of Carbonyl Group: Overview
This Topic covers sub-topics such as Carbonyl Groups, Aldehydes and Ketones, Structure of Carbonyl Group, Nomenclature of Aldehydes and Ketones, Common Names of Aldehydes and Ketones and, IUPAC Names of Aldehydes and Ketones
Important Questions on Nomenclature and Structure of Carbonyl Group
The IUPAC name of the following compound is:
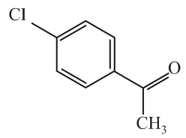
The name of the following organic compound would be:
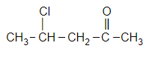
How many structural isomers of butanal are possible? (Other than butanal)
The carbonyl functional group showing metamerism is
What is the hybridisation of the carboxyl carbon atom of carboxylic acids?
Identify a ketone molecule, among the given compounds.
A carbonyl compound muscone finds application in
Identify a compound not having the carbonyl group.
Explain the structure of the carbonyl group.
The carbon-oxygen double bond in a carbonyl group is polar due to
Aldehydes can be represented by the general formula
Which isomerism is shown by pentanone?
Total number of carbonyl compounds represented by the formula are _____.(Excluding stereoisomers)
Which of the following will not support Aldol condensation?
Which of the following functional groups is generally least reactive towards nucleophilic substitution reactions?
In how many of the following reactions, aldehyde is formed as a product?
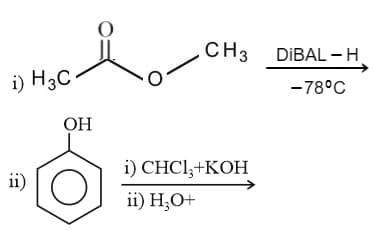
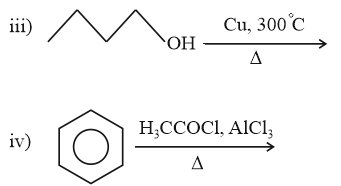
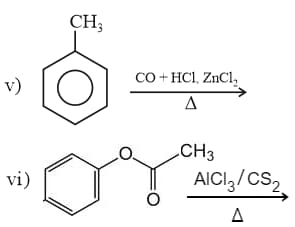
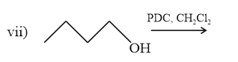
IUPAC name of is
Ethanedial has which functional group(s)?
Which of the following statements is true about propanone with respect to bonds,bonds and lone pairs of electrons, respectively?
In , there are non-bonding electron pairs, and and are -
