Temperature Dependence of the Rate of a Reaction
Temperature Dependence of the Rate of a Reaction: Overview
This topic consists of various concepts like Effect of Temperature on Rate of a Reaction,Temperature Coefficient,Graphical Representation of Rate Constant versus Temperature, etc.
Important Questions on Temperature Dependence of the Rate of a Reaction
The rate of a reaction escalates four times when the temperature changes from to Determine the energy of activation of the reaction, assuming that it does not change with temperature?
The minimum energy required to start a chemical reaction is known as:
The activation energy for the reaction is at . The fraction of molecules having energy equal to or greater than activation energy is:
For a decomposition reaction the values of rate constant k at two different temperatures are given below :
The value of activation energy for this reaction is:
For a hypothetical reaction , the activation energy for forward and backward reactions are and respectively. The heat of reaction is
From the following data
Which of the following is the appropriate of the reaction?
The plot of versus for a reversible reaction is shown.
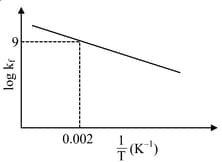
Pre-exponential factors for the forward and backward reactions are and , respectively. If the value of for the reaction at is , the value of at is
equilibrium constant of the reaction, rate constant of forward reaction, rate constant of backward reaction]
Given below are two statements: one labelled as Assertion A and other is labelled as Reason R:
Assertion A: A reaction can have zero activation energy
Reason R: The minimum extra amount of energy absorbed by reactant molecules so that their energy becomes equal to threshold value, is called activation energy.
For the adsorption of hydrogen on platinum, the activation energy is and for the adsorption of hydrogen on nickel, the activation energy is The logarithm of the ratio of the rates of chemisorption on equal areas of the metals at is (Nearest integer)
Given:
The number of given statement/s which is/are correct is_____
(A) The stronger the temperature dependence of the rate constant, the higher is the activation energy.
(B) If a reaction has zero activation energy, its rate is independent of temperature.
(C) The stronger the temperature dependence of the rate constant, the smaller is the activation energy.
(D) If there is no correlation between the temperature and the rate constant then it means that the reaction has negative activation energy.
Consider the following reaction that goes from to in three steps as shown below:
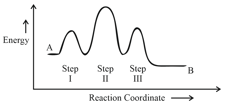
Choose the correct option
| Number of Intermediates |
Number of Activated Complexes |
Rate determining step |
|
The rate constant for the reaction: are
Calculate activation energy of the reaction.()
The rate constants of a reaction at are respectively. What extra piece of information is needed to calculate the value of (frequency factor)?
(According to the Arrhenius equation, rate constant is given by, )
Kamlesh was conducting an experiment to figure out the rate equation of the following reaction:
He measured the rate of this reaction as a function of initial concentrations of the reactants as follows:
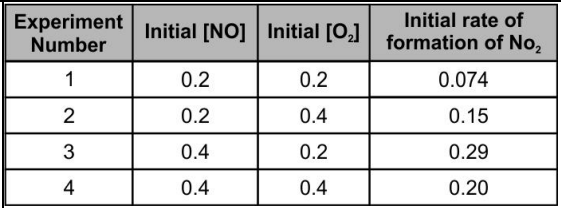
Which of the following could be a reason for the inconsistency in the initial rate of formation of data for experiment ?
Draw the graphical representation of arrenhious equation.
If rate of reaction increases by a factor of 2, when temperature is raised from . What is the value of activation energy in kJ/mol? By what factor does rate of reaction increase if temperature is increased from
(a) If temperature coefficient is same in given temperature range
(b) If temperature coefficient is function of temperature.
If the definition of the temperature coefficient of the reaction holds good for a reaction between and , the activation energy for the reaction in is
The rate constant of a reaction is increased times after addition of catalyst to the reaction mixture at the same temperature of . The change in the activation energy of this reaction is
(Take )
The potential energy diagram for four reactions are given below
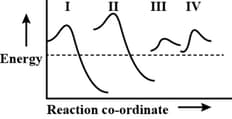
Which one of the following statements about these diagrams is incorrect?
For the reaction under the same concentration conditions of the reactants, the rate of reaction at IS 1500 times as fast as the same reaction at . Calculate the activation energy of the reaction.If the frequency factor is Calculate the rate constant of the reaction at .
