Kohlrausch's Law and its Application
Kohlrausch's Law and its Application: Overview
This Topic covers sub-topics such as Kohlrausch's Law, Applications of Kohlrausch's Law, Molar Conductivity at Infinite Dilution and, Determination of Molar Conductivity of a Weak Electrolyte at Given Concentration
Important Questions on Kohlrausch's Law and its Application
The law that states about independent migration of ions is:
Find the solubility product of a saturated solution of at 298 K in water if the emf of the cell is 0.164 V at 298 K.
| Electrolyte | |||||
Calculate at infinite dilution in at :
Plotting against for aqueous solutions of a monobasic weak acid resulted in a straight line with axis intercept of and slope of . The ratio is
Form the following molar conductivities at infinite dilution,
Calculate for
Which amongst the following expressions are not correct?
The molar conductivity of at infinite dilution is . Using the graph and given information, the molar conductivity of will be:
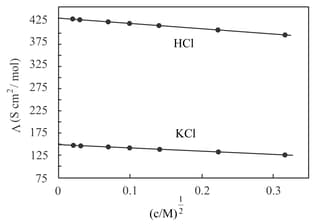
The molar conductance of acetic acid at infinite dilution is . If the conductivity of acetic acid is , the apparent degree of ionization is
Following limiting molar conductivities are given as
(H2SO4)= x Scm² mol-1
(MgSO4)=- y Scm² mol-1
((CH3COO)2Mg) = z Scm² mol-1
(In Scm² mol-1) for CH3COOH will be
Following limiting molar conductivities are given as:
(in )
for will be
How is the molar conductivity of a weak electrolyte at infinite dilution determined?
Applying Kohlrausch law of independent migration of ions, write the expression to determine the limiting molar conductivity of calcium chloride
State Kohlrausch law of independent migration of ions.
The equivalent conductance at infinite dilution of the salt is . If the transport number of is , calculate the ionic mobility of the ion.
The molar conductivity of acetic acid solution at infinite dilution is . Calculate the molar conductivity of acetic acid solution, given that the dissociation constant of acetic acid is .
How does Kohlrausch's law help in the calculation degree of dissociation of a weak electrolyte?
Define Kohlrausch's law. How does it help in calculation of for a weak electrolyte?
Define Kohlrausch's law. How can it be used to find the degree of dissociation of a weak electrolyte?
Explain Kohlrausch law. (Definition and Formula)
Conductivity of acetic acid solution is . Calculate its molar conductivity in this solution. If for acetic acid be , what would be its dissociation constant?
