Enthalpy Changes in Solution
Enthalpy Changes in Solution: Overview
This Topic covers sub-topics such as Dissolution, Enthalpy of Neutralization, Hydration Energy, Solvation Energy, Standard Enthalpy of Solution, Factors Affecting Hydration Energy and, Determination of Enthalpy of Neutralization
Important Questions on Enthalpy Changes in Solution
The absolute enthalpy of neutralisation of the reaction:
will be:
[Consider the actual value instead of magnitude].
Define dissolution.
Describe the method to determine the enthalpy of neutralization.
Select whether the following statement is True or False:
The standard enthalpy of hydration, , for an ion is defined as the enthalpy change when mol of the gaseous ion is added to a solvent other than water, to form a dilute solution.
Define standard enthalpy of hydration for a substance.
If the hydration energy is equal to or greater than the lattice energy, then an ionic solid is water-soluble.
Explain the conditions for the solubility of an ionic solid in terms of lattice energy and hydration energy.
Solvation energy can be defined as the change in Gibbs energy of a solvent when a solute is dissolved in that solvent.
Define solvation energy.
| Column I (Solutions mixed) | Column II (Heat evolved) |
| (A) 500 mL of 0.1 M HCI acid + 200 mL of 0.2 M NaOH solution | (p) 4568 J |
| (B) 200 mL of 0.2 M + 400 mL of 0.5 M KOH solution | (q) 2284 J |
| (C) 500 mL of 0.1 M HCl + 500 mL of 0.1 M | (r) 2760 J |
| (D) 500 mL of 0.1 M acetic acid + 500 mL of 0.1 M NaOH | (s) 2575 J |
Matching Type Question
Which of the followings show apparently low heat of neutralisation:
Which is the correct order of ionic mobility in aqueous medium?
The enthalpy of neutralization of and is and enthalpy of neutralisation of with a strong acid is . The enthalpy of ionization of will be
Given,
Enthalpy change when mole of , a strong base, is completely neutralized by is ( of neutralization of strong acid with strong base is = )
Which of the following is not a correct statement about enthalpy of solution?
Study the figure given below and mark the correct expression.
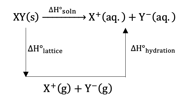
The enthalpy of solution of In water can be determined by
What would be the heat released in , when an aqueous solution containing is mixed with ?
(Enthalpy of neutralization is )
Report your answer by rounding it up to the nearest whole number.
The heat evolved during the formation of of hydrated copper sulphate from anhydrous copper sulphate (mol.wt = 159.5) is
The heat of neutralization of a strong acid by a strong base is . The heat released when 0.5 mol is mixed with 0.2 mol KOH in aqueous medium is
The enthalpies of neutralization and a strong base by are and respectively. When one mole of is added to a solution containing mole of and mole of , the enthalpy change was . In what ratio is the acid distributed between and respectively.
