Solubility of a Solid in a Liquid,
Solubility of a Solid in a Liquid,: Overview
This Topic covers sub-topics such as Solubility of a Solid in a Liquid, Effect of Temperature on Solubility of a Solid in a Liquid, Effect of Pressure on Solubility of a Solid in a Liquid and, Effect of Nature of Solute on Solubility of a Solid in a Liquid
Important Questions on Solubility of a Solid in a Liquid,
In which solution solubility ofis less than that in pure water?
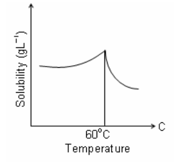
Solubility curve of a hydrated salt in water with temperature is given. The curve indicates that the solution process is
Maximum amount of a solid solute that can be dissolved in a specified amount of a given liquid solvent does not depend upon _____
(i) Temperature
(ii) Nature of Solute
(iii) Pressure
(iv) Nature of Solvent
is soluble in . The solubility is due to the formation of
When is dissolved in water, the temperature of the solution decreases. Which of the following statements describes the effect of increase in temperature on the solubility of in water?
On dissolving sugar in water at room temperature, solution feels cool to touch. Under which of the following cases dissolution of sugar will be most rapid?
Which of the following solvent can easily dissolve in it?
Which of the following are non-polar solvents?
The Maximum amount of a solid solute that can be dissolved in a specified amount of a given liquid solvent does not depend upon _____.
Dissolution of solids in water can be exothermic or endothermic process but gases dissolve in water always with the evolution of heat. Dissolution of a substance in water can be either because ion dipole interactions or by hydrogen bond formation. Pressure plays a significant role on the solubility to gases in water. Solubility of a gas in terms of mol fraction is related to pressure according to the mathematical relation
On the basic of above paragraph answer the following questions.
When a pinch of salt is added to a freshly opened bottle of cocacola or limca, a lot of effervescence occurs with evolution of a colourless gas
Cotyledons are also called-
Arrange the following in order of decreasing solubility in .
Phenol (i), toluene (ii), chloroform (iii)
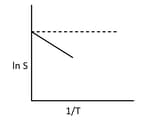
The solubility of a solute in water varies with temperature as given by, being the enthalpy of the solution. For a given solute, the variation of with temperature is as shown in the figure. The solute is expected to be