Adsorption and Absorption
Adsorption and Absorption: Overview
This Topic covers sub-topics such as Absorption, Adsorption, Adsorption Isotherms, Freundlich's Adsorption Isotherm, Adsorbent, Desorption, Physical Adsorption, Adsorbate, Mechanism of Adsorption, Chemical Adsorption and, Applications of Adsorption
Important Questions on Adsorption and Absorption
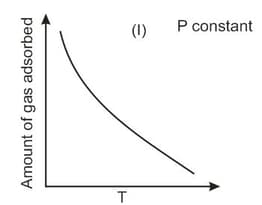
The given graphs/data I, II, III and IV represent general trends observed for different physisorption and chemisorption processes under mild conditions of temperature and pressure. Which of the following choice(s) about I, II, III and IV is (are) correct?
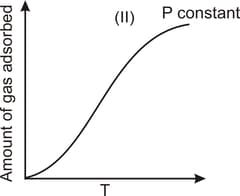
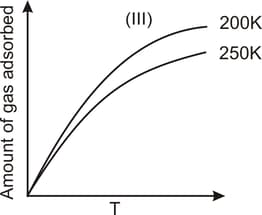
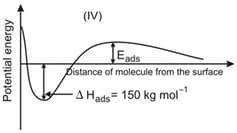
Physisorption arises because of
Among physisorption and chemisorption, which type of a adsorption has a higher enthalpy of adsorption?
20% of surface sites are occupied by molecules. The density of surface sites is and total surface area is . The catalyst is heated to 300 K while is completely desorbed into a pressure of 0.001 atm and volume of . The number of active sites occupied by each molecule will be
20% of surface sites are occupied by molecules. The density of surface sites is and total surface area is . The catalyst is heated to 300 K while is completely desorbed into a pressure of 0.001 atm and volume of . The number of active sites occupied by each molecule will be:
Adsorption of gases on solid surface is generally exothermic because:
Rate of physisorption increase with
Rate of physisorption increase with:
Identify the suitable conditions when the adsorption equilibrium is attained.
The wrong statement about Freundlich isotherm is/are
A) It can be represented at high pressure as
B) The slope of the straight line of '' Vs ', is ''
C) It explains the adsorption behaviour in an approximate manner
D) It holds good in the pressure range to
Predict the signs for entropy and enthalpy of adsorption.
In the adsorption process of acetic acid in activated charcoal, acetic acid is
For a linear plot of log versus log in a Freundlich adsorption isotherm, the correct statement is ( and are constants)
The thermodynamic condition for the process of adsorption is
The variation of physical adsorption with temperature is shown by
Which of the following curve is in accordance with Freundlich adsorption isotherm?
The enthalpy of chemical adsorption is _____ than the enthalpy of physical adsorption.
What is the amount of heat released in a chemical adsorption?
The plot that best represents the relationship between the extent of adsorption and pressure is
The role of pine oil used in froth floatation process is