Applications of First Law of Thermodynamics
Applications of First Law of Thermodynamics: Overview
This Topic covers sub-topics such as Work Done in Reversible Adiabatic Process, Graph of Adiabatic Process, Graph of Isobaric Process, Work Done in Irreversible Adiabatic Process, Graph of Isothermal Process and, Graph of Isochoric Process
Important Questions on Applications of First Law of Thermodynamics
The work done during the expansion of a gas from a volume of against a constant external pressure of 3 atm is (1 L atm = 101.32 J):
moles of an ideal gas expands isothermally against a constant pressure of Pascal from L.The amount of work involved is
One mole of an ideal gas is taken from and along two paths denoted by the solid and the dashed lines as shown in the graph below. If the work done along the solid line path is and that along the dotted line path is then the integer closest to the ratio is:
The change in internal energy equals
The magnitude of reversible work done by an ideal gas in four different processes: isothermal expansion, adiabatic expansion, constant pressure expansion, and free expansion are , and respectively. Choose the right order of sequence for the magnitude of the work done. (Change in the volume is same for all the processes.)
A thermodynamic cycle in the pressure -volume plane is given below:
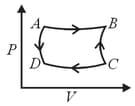
and are isothermal processes while and are adiabatic processes. The same cycle in the temperature - entropy plane is :
One mole of an ideal gas (at ) is expanded from volume to at constant temperature. The value of work done (in ), if gas is expanded against vacuum ?
Among the following, which expression is most accurate to calculate the work done by gas in reversible isothermal expansion?
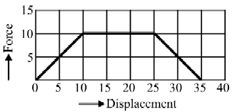
Adjacent figure shows the force-displacement graph of a moving body, the work done in displacing body from to by the shown force is equals to
An ideal gas is taken around the cycle ABCA as:
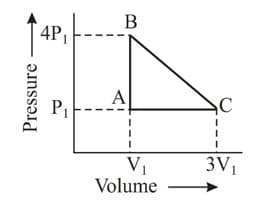
Calculate work done in the cyclic process?
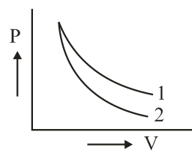
P-V plots for two gases during adiabatic expansion are shown in figure. Plots 1 and plots 2 should correspond respectively to
Two moles of a gas expand reversibly and isothermally at temperature of Initial volume of the gas is while the final pressure is The work done by gas is
For an isothermal reversible expansion process, the value of can be calculated by the expression
Which of the following P-V diagram represent the graph of isochoric process?
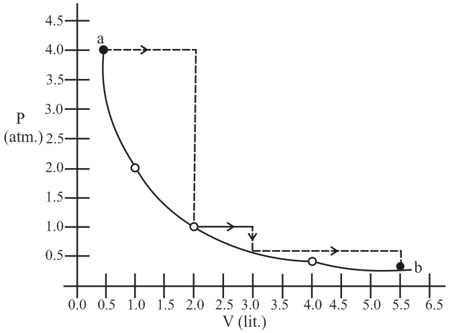
A system consisting of 1 mol of an ideal gas undergoes a reversible process, (schematically indicated in the figure below). If the temperature at the starting point A is 300 K and the work done in the process is 1 L atm, the heat exchanged in the entire process in L atm is
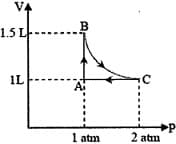
For a certain process, the pressure of a diatomic gas varies according to the relation , where a is a constant. The molar heat capacity of the gas for this reaction is.
The slope of isothermal and adiabatic curves are related as
Which of the figures given below show the adiabatic process?
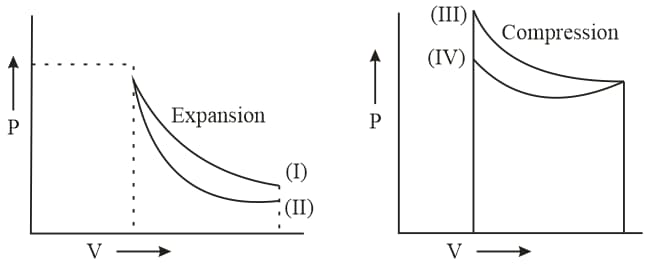
Assertion: Work done by a gas in reversible isothermal expansion is more than the work done by the gas in reversible adiabatic expansion if final volume is same.
Reason: Temperature remains constant in isothermal expansion, and not in adiabatic expansion.
Calculate Q and w for the isothermal reversible expansion of one mole of an ideal gas from an initial pressure of to a final pressure of at a constant temperature of .
