Solubility of Salts
Solubility of Salts: Overview
This Topic covers sub-topics such as Salts, Chromatography, Paper Chromatography, Column Chromatography, Filtration, Double Displacement Reactions, Principle of Chromatography, Precipitation Reactions and, Principle of Column Chromatography
Important Questions on Solubility of Salts
The technique used to separate coloured pigments from a mixture is column chromatography.
State the principle involved of chromatography?
Which of the following nitrate is an insoluble salt?
Ionic compounds are insoluble in _____ (polar/non polar) solvents.
Water that flows through rivers may have compounds dissolved in it that make it unsafe to drink. Which technique could be used to remove the dissolved substances and obtain pure water?
Label the figure given below.
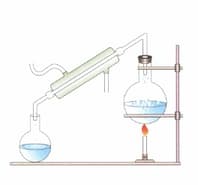
Where and why is it used?
Label the figure given below.
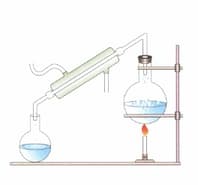
What is the process called?
State whether the following statement is True or False. Correct the false statement.
Formation of precipitate is a characteristic feature of a chemical reaction.
Which of the following is formed when lead acetate reacts with potassium iodide?
In a double displacement reaction, one substance breaks to form two new products.
In a precipitation reaction of lead acetate and potassium iodide, a blue-coloured precipitate is formed.
Chromatography is used to separate mixture of chemicals in their liquid or gaseous form.
State whether the following statements are True or False.
The process to obtain pure substance from a mixture in a solution is called distillation.
What is the principle used in distillation?
On what principle does chromatography work?
The pattern obtained on chromatography paper is called _____.
(Chromatograph, Valency, Cuprum, Negatively, , one, , Positively, Separating funnel)
Which mixture can be separated using distillation?
Distillation is the process by which a pure substance is obtained from a mixture.
