Principle of Calorimetry
Principle of Calorimetry: Overview
This topic consists of various concepts like Calorimetry,Units of Heat,Mechanical Equivalent of Heat, etc.
Important Questions on Principle of Calorimetry
For shown situation, calculate the temperature of the common interface.
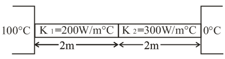
There are three liquids and . The mass, heat capacity and temperature for liquid is , and , for liquid is , and and for liquid is , and respectively. If all the three liquids are mixed, then final temperature would be
A continuous flow water heater (geyser) has an electrical power rating and efficiency of conversion of electrical power into heat . If water is flowing through the device at the rate of , and the inlet temperature is , the outlet temperature will be
What will be the final temperature if of water at is added to of water at ?
If the Ropes of masses & are Replaced by the Massless Strings of Similar Corresponding Lengths. The String attached to masses is cut at the point Just above the mass A Machine which converts Mechanical Energy into Heat Energy is placed below the (Rope of) Mass before the Ropes are replaced. Efficiency of machine is . The Heat is supplied to a Container which consists of of Ice at . Find the Final Temp. & composition Pn the container?

Suppose the masses of the calorimeter, the water in it and the hot object made of copper which is put in the calorimeter are the same. The initial temperature of the calorimeter and water is and that of the hot object is . The specific heats of copper and water are and respectively. What will be the final temperature of water?
You are provided some lead shots. How do you find the specific heat of lead?
Initially, a beaker has of water at temperature . Later another of water at temperature was poured into the beaker. The temperature, of the water after mixing is
A thermally insulated vessel contains some water at . The vessel is connected to a vacuum pump to pump out water vapour. This results in some water getting frozen. It is given latent heat of vaporization of water at and latent heat of freezing of water . The maximum percentage amount of water that will be solidified in this manner will be:-
Why the container of calorimeter is made up of copper?
What is calorimeter?
In a certain experiment, liquid helium at an initial temperature of is to be further cooled by bringing it into contact with a paramagnetic salt which is initially at temperature . The heat capacity of liquid helium is expressed as and that of salt is expressed as . Assume that the mixture is thermally and mechanically isolated and no work is done during the process. If the final temperature of the system is , the initial temperature of the salt is
water of specific heat is kept in a container at . If of ice at is required to cool down the water from to , then what is the water equivalent of the container? (Latent heat of fusion for ice )
What will be the maximum percentage amount of water that solidifies, if a thermally insulated vessel contains some water at ? The vessel is connected to a vacuum pump to pump out water vapour. This results in some water getting frozen. It is given Latent heat of vaporization of water at and latent heat of freezing of water . Give answer in the nearest integer.
Two blocks of ice when pressed together join to
form one block. It happens because
When inflated tyre bursts, the air escaps out.
When salt is properly mixed with ice, the melting point of ice?
A circular disc of copper has a symmetrical hole at its centre. The disc is uniformly heated. The diameter of the hole will
Ice in a freezer is at of this ice is mixed with of water at . Take the freezing temperature of water to be , the specific heat of ice equal to specific heat of water equal to and the latent heat of ice equal to .
Assuming no loss of heat to the environment, the mass of ice in the final mixture is closest to
of copper is heated to increase its temperature by If the same quantity of heat is given to of water, the rise in temperature is (specific heat of and specific heat of water is
