General Terms in Thermodynamics
General Terms in Thermodynamics: Overview
This topic consists of various concepts like General Terms in Thermodynamics,System, Surroundings and Boundaries,Thermodynamic System, etc.
Important Questions on General Terms in Thermodynamics
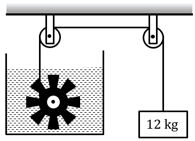
The figure shows a peddle wheel couple to a mass of through fixed frictionless pulley. The paddle is immersed in a liquid of heat capacity kept in an adiabatic container. Consider a time interval in which the block falls slowly through.
(a) How much heat is given to the liquid ?
(b) How much work is done on the liquid?
(c) Calculate the rise in the temperature of the liquid neglecting the heat capacity of the container and the paddle
.
Time taken to heat water upto a temperature of (from room temperature) is and time taken to heat mustard oil (of same mass and at room temperature) upto a temperature of is , then (given mustard oil has smaller heat capacity)
What is the thermodynamic system?
For the process to occur under adiabatic conditions, the correct condition is:
Which is not the microscopic variable of the system?
What are the macroscopic variables of a thermal system?
What is an isolated system. Give its one example.
What are the constituents of a thermodynamic system?
A system is said to be an isolated system if
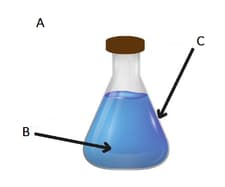
If we want to study the liquid inside the flask, we can define A, B and C as
What is a boundary for a thermodynamic system?
What do you understand by macroscopic parameters in thermodynamics?
Explain System and Boundary.
Define surrounding in thermodynamic system physics
What Are The Types Of Boundaries In Thermodynamics
For an isolated system, the entropy:
Which among this is not an exothermic reaction?
What is the equation of entropy?