Rutherford's Model of Atom
Rutherford's Model of Atom: Overview
This topic covers concepts such as interpretation of Rutherford experimental results and Rutherford model of atom.
Important Questions on Rutherford's Model of Atom
Mention the conclusion of the Geiger-Marsden -particle scattering experiment.
In the ground state in electrons are in stable equilibrium while in electrons always experience a net force. Here, and refer to
List the drawbacks of Rutherford's model of atom.
Explain briefly how the Rutherford atomic model is not able to explain stability of the atom.
According to the rutherford's model, entire positive charge is concentrated in the centre of the atom.
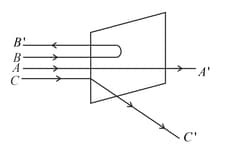
A beam of fast moving alpha particles were directed towards a thin film of Gold. The parts and of the transmitted and reflected beams corresponding to the incident parts and of the beam are shown in the adjoing diagram. The number of alpha particles in
Rutherford proposed a nuclear model of the atom in which the atom is composed of a tiny nucleus at its edge containing positive charges.
The significant result deduced from the Rutherford's scattering experiment is that
Explain the Rutherford's model of an atom.
What were the shortcomings of Rutherford model? Explain in detail how Bohr removed them in his model.
Mention the main consideration in Rutherford's atomic model.
Rutherford -scattering demonstrates the existence of which of the following ?
What is the conclusion of Rutherford’s alpha particle scattering experiment ?
An particle after passing through a potential difference of volts collides with a nucleus. If the atomic number of the nucleus is then the distance of closest approach of particle to the nucleus will be
According to particle scattering experiment of Rutherford.
Here no. of α-particle scattered at an angle
Kinetic energy (initial) of particle.
What is the value of
Which of the following is incorrect regarding Rutherford's atomic model?
Which of the following is incorrect regarding Rutherford's atomic model?
In Rutherford scattering experiment, what will be the correct angle for scattering for an impact parameter ?
Rutherford's particle experiment shows that the atoms have
