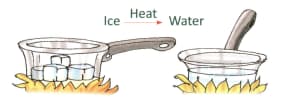
Changes of State
Changes of State: Overview
This Topic covers sub-topics such as Condensation, Sublimation, Evaporation, Boiling Point of Water, Crystallisation, Crystallisation of Copper Sulphate, Vapourization, Fusion of Ice and, Extraction of Salt from Sea Water
Important Questions on Changes of State
Look at the picture carefully and answer the following questions.
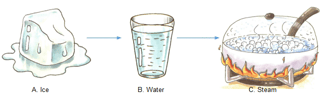
The process by which A changes into B.
Fill in the box with words from the box:
| melts | hardens | evaporates | stretches | dissolves |
Water _____ on heating.
How is evaporation different from sublimation?
Which type of clothes absorbs the sweat easily thereby allowing it to evaporate easily?
It is a hot summer day,Priyanshi and Ali are wearing a cotton and a nylon clothes respectively.Who do you think would be more comfortable?
Identify the phenomenon responsible for the spread of disease spreading germs when we sneeze.
The phenomenon of change of a liquid into the gaseous state at any temperature below its boiling point is called _____.
Evaporation of a liquid at room temperature leads to a _____ effect.
Which of the following conditions will increase the evaporation of water?
During summer, _____ helps to keep the water in an earthern pot cool.
During summer, water kept in an earthen pot so that it remains cool. The water becomes cool because of the phenomenon of
If the surface area is increased, the rate of evaporation .
Change of liquid state to gases state is known as _____.
Sodium chloride can be separated from water by the physical process of _____.
Salt can be separated from its solution (salt dissolved in water) because
Place an ice cube on a plate for 10 minutes. What did you notice? Is there any change in the ice cube?
Write the state of the matter in the following situation.
Vapour on cooling.
Write the state of matter in the following situation.
Water on heating