Chemical Changes and Equations
Chemical Changes and Equations: Overview
This topic covers concepts, such as, Chemical Reactions, Reaction between Magnesium and Oxygen, Skeletal Chemical Equations & Catalyst etc.
Important Questions on Chemical Changes and Equations
For the following reaction, identify the products formed and balance the equation:
For the following reaction, identify the products formed and balance the equation:
For the following reaction, identify the products formed and balance the equation:
For the following reaction, identify the products formed and balance the equation:
For the following reaction, identify the products formed and balance the equation:
Balance the following chemical equation:
Balance the chemical equation:
Balance the chemical equation:
Balance the chemical equation:
Balance the chemical equation:
Balance the chemical equation:
Explain the types of reaction?
Balance the equation:
Balance the given chemical reaction:
Find the number of phosphine molecules formed when molecules of reacts with water. Slaked lime is another product.
During balancing equation can there be different number for balancing both sides?
A piece of magnesium ribbon is added to a flask containing dilute hydrochloric acid.
Hydrogen gas is formed which is collected in the measuring cylinder.
The line on the graph indicates the rate of chemical reaction occurring in the flask.
The amount of hydrogen formed with time is plotted on a graph.
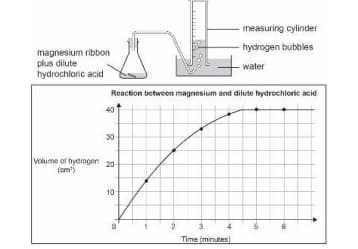 .
.
Which of these could increase the rate of reaction in the flask?
Circle ‘Yes’ or ‘No’ for each row.
| Will this increase the rate of reaction? | Yes or No |
| Adding more acid to the flask | Yes/No |
| Heating the acid in the flask | Yes/No |
| Using a higher concentration of acid | Yes/No |
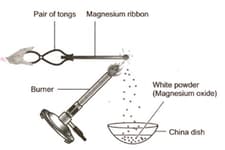
Which of the following is incorrect observation of the above reaction?
What are chemical reactions?
Does hydrogen gas reduce magnesium oxide?
