Solution
Solution: Overview
This Topic covers sub-topics such as Solution, Solubility, Solvent, Saturated Solution, Solute, Concentration of a Solution, Concentrated Solution, True Solution, Unsaturated Solution, Dilute Solutions, Properties of Solution and, Sugar and Water Solution
Important Questions on Solution
Pratiksha dissolved of copper sulphate in of water at . She stirred the solution thoroughly and after filtering it of copper sulphate was obtained as the residue. In order to dissolve the obtained residue in the above solution at the same temperature, the amount of water that should be added by her will be
What happens to the cold drinks when we keep it in open for sometime
If the density of water is then find its molarity.
In ml of water, g of glucose and g of common salt is dissolved. Calculate the concentration of:
(a) Glucose and
(b) common salt in terms of mass by a mass percentage of the solution. (Density of water is )
Put a drop of blood in three types of liquids:
- Pure water
- Salt solution
- Water containing glucose and .
What will happen to the blood drop and why? Explain your answer.
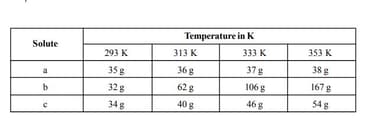
Seema took three solids, , , and and made their saturated solutions in water at different temperatures. The amounts of , and used to form a saturated solution in water at different temperatures. The amounts of , , and used to form saturated solutions are shown in the table:
What is the concentration in mass percent of and at respectively?
Seema took three solids, , , and and made their saturated solutions in water at different temperatures. The amounts of , and used to form a saturated solution in water at different temperatures. The amounts of , , and used to form saturated solutions are shown in the table:
A solution that has a relatively small amount of dissolved solute is called as:
A _____ solution is a one that has only a little solute dissolved in a certain amount of solvent.
What do you understand by dilution? Explain what is a dilute solution.
Which of the following is not an example of dilute solution?
Define solution. Write the characteristics of the solution.
Which of the following non-metal is used in the purple-coloured antiseptic solution?
Name the technique for the separation of the following:
Copper sulphate crystals from its aqueous solution. (crystallisation/ winnowing/ distillation)
Answer in one or two words.
Aerated drinks contain this gas.