The Structure of an Atom
The Structure of an Atom: Overview
This topic covers concepts, such as, The Unknown Structure of Atom, Structure of an Atom, Symbol of Neutron & Alpha Particles etc.
Important Questions on The Structure of an Atom
Explain how the Bohr's atomic model was contradictory to Heisenberg's uncertainty principle.
Describe J. J. Thomson's atomic model.
According to Rutherford's atomic model, where are the protons and electrons located in an atom?
If Rutherford's atomic model is correct, then the atom should collapse. Why?
What are orbits and why are they called stationary orbits?
How many orbitals are there in and shells? What are they?
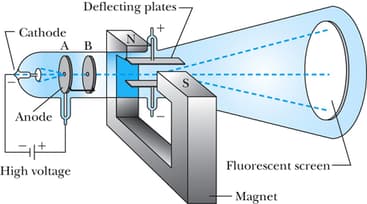
If the given schematic diagram represents Thomson's experiment and the corresponding observation, what would be his atomic model?
ray scattering experiment proved that the positive particles are present in the extra nuclear part of an atom.
What happens when an electron jumps from a lower energy level to a higher energy level?
How Rutherford came to a conclusion that electrons revolve around the nucleus?
Which of these is present inside the nucleus of an atom?
What is the symbol of neutron?
(A) (B) (C)
Enter your correct answer as A, B or C.
The symbol of neutron is _____.
The symbol of the neutron is 'n'.
What is the symbol of neutron?
What was the conclusion given by Rutherford , after performing α-particle scattering experiment ?
The structure of atom was unknown for long time and early Indian and Greek philosophers who were of the view that atoms are the fundamental building blocks of matter. According to them, the continued subdivisions of matter would ultimately yield atoms which would not be further divisible speculations and there was no way to test them _____ (experimentally / mentally)
According to JJ thomson,"The negative and positive charges are equal in magnitude. So, the atom as a whole is electrically _____ .
An atom consists of a positively charged sphere and the _____ are embedded in it.
What is JJ Thomson model of atom?
