Valency
Valency: Overview
This topic covers concepts, such as, Valency, Valencies of Noble Gases, Octet Rule, Calculation of Valency of Elements & Valency of the First 18 Elements etc.
Important Questions on Valency
Can all elements form both electro valency and covalency?
How do we find the valency of the elements in a compound, if three elements are present in a compound eg
The valency of oxygen is in the water molecule.
What is the general name of the elements having electrons in the valence shell of their atoms?
Choose from the following list, as to what matches the descriptions below:
A cation whose electronic configuration resembles argon.
Enter your answer as or
Give reasons for the following :
Noble gases show least reactivity.
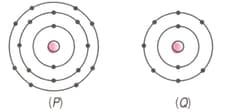
Find out the valency of the atoms represented by the figure.
Match the column I with column II and select the correct answer by choosing an appropriate option.
| Column I | Column II |
| P. Fractional atomic weight | 1. Hydrogen |
| Q. for | 2. Chlorine |
| R. Variable valency | 3. |
| S. Number of neutrons | 4. |
The valency shown by an element having atomic number is
An element (atomic no. ) combines with another element whose electronic configuration is . What will be the formula of the compound formed?
How will you find the valency of aluminium, oxygen and fluorine?
What is the cause of the chemical combination? How is the number of valence electrons related to the combining capacity of the atom?
Define valency by taking an example of magnesium Atomic number and oxygen Atomic number .
The electronic configuration of is . Why is the valency of oxygen and not ?
Define valency. What conclusions can be drawn about the reactivity of an atom from its valency?
