Fundamental Laws of Gases
Fundamental Laws of Gases: Overview
This Topic covers sub-topics such as Boyle's Law, Charles's Law, Avogadro's Law and, Fundamental Laws of Gases
Important Questions on Fundamental Laws of Gases
Choose the fundamental gas law represented by the equation, from the following options.
Boyle's law states that when the temperature of a gas is kept constant, the volume of a fixed mass of gas is inversely proportional to its pressure.
When a large bubble rises from the bottom of a lake to the surface, its radius doubles. If atmospheric pressure is equal to that of a column of water of height . The depth of the lake is
Average volume available to a molecule in a sample of a gas at STP is
The volume of a water molecule is (Take, density of water is and Avogadro's number )
At a constant pressure, of the following graphs the one which represents the variation of the density of an ideal gas with the absolute temperature , is
Assertion: A gas can be liquefied at any temperature by increase of pressure alone.
Reason: On increasing the pressure the temperature of gas increases at constant volume.
A perfect gas at is heated at constant pressure so as to double its volume. The increase in temperature of the gas will be
A certain mass of a gas occupies a volume of at STP. To what temperature the gas must be heated to double its volume, keeping the pressure constant?
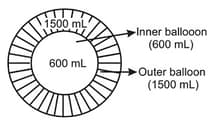
Two inflated balloons (I) and (II) having volume 600 mL and 1500 mL at 300 K are taken as shown in diagram. If maximum volume of inner and outer balloons are 800 mL and 1800 mL respectively then, find the balloon which will burst first on heating ?
An ideal gas is initially at temperature T and volume V. Its volume is increased by due to an increase an increase in temperature , pressure remaining constant. The physical quantity varies with temperature are
Volume-temperature graph at atmospheric pressure for a monoatomic gas ( is
