A constant pressure thermometer when immersed in ice-cooled water gives volume reading unit and when immersed in boiling liquid, it gives a reading of unit. What is the boiling point of the liquid?

Important Questions on Kinetic Theory of Gases
One mole of a monatomic ideal gas is taken along two cyclic processes and as shown in the diagram. The processes involved are purely isochoric, isobaric, isothermal or adiabatic.
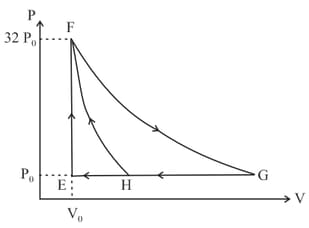
Match the paths in List I with the magnitudes of the work done in List II and select the correct answer using the codes given below the lists.
Assertion: An ideal gas is enclosed within a container fitted with a piston. When volume of this enclosed gas is increased at constant temperature, the pressure exerted by the gas on the piston decreases.
Reason: In the above situation, the rate of molecules striking the piston decreases. If the rate ạt which molecules of a gas having same average speed striking a given area of the wall decreases, the pressure exerted by gas on the wall decreases.
Assertion: Different gases at the same conditions of temperature and pressure have same root mean square speed.
Reason: Average K.E. of gas is inversely proportional to temperature in Kelvin.
