A double charged lithium atom is equivalent to hydrogen atom whose atomic number is . The wavelength of required radiation for emitting electron from first to third Bohr orbit in will be? (Ionisation energy of hydrogen atom is )

Important Questions on Atomic Physics
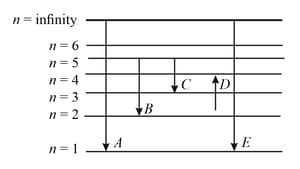
The energy levels of the hydrogen spectrum is shown in the figure. There are some transitions ,, , and . Transition , and respectively represent
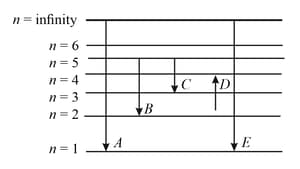
In the given figure and respectively represent
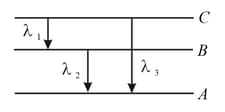
 ,
,
The energy level diagram for a hydrogen-like atom is shown in the figure. The radius of its first Bohr orbit is

The following diagram indicates the energy levels of a certain atom when the system moves from level to . A photon of wavelength is emitted. The wavelength of photon produced during its transition from level to is . The ratio will be
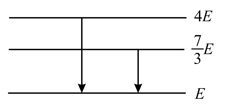
Energy levels , , of a certain atom corresponding to increasing values of energy, i.e., . If , and are the wavelengths of radiations corresponding to the transitions to , to and to respectively, which of the following statements is correct