HARD
JEE Main
IMPORTANT
Earn 100
In a hypothetical Bohr hydrogen, the mass of the electron is doubled. The energy, and the radius, of the first orbit, will be? ( is the Bohr radius)
(a)
(b)
(c)
(d)
29.41% studentsanswered this correctly

Important Questions on Atomic Physics
HARD
JEE Main
IMPORTANT
Which of the following transitions in a hydrogen atom emits photon of the highest frequency?
HARD
JEE Main
IMPORTANT
The following diagram indicates the energy levels of a certain atom when the system moves from level to , a photon of wavelength is emitted. The wavelength of photon produced during its transition from level to is
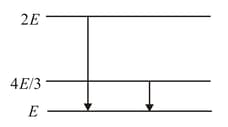
HARD
JEE Main
IMPORTANT
A double charged lithium atom is equivalent to hydrogen atom whose atomic number is . The wavelength of required radiation for emitting electron from first to third Bohr orbit in will be? (Ionisation energy of hydrogen atom is )
MEDIUM
JEE Main
IMPORTANT
The energy levels of the hydrogen spectrum is shown in the figure. There are some transitions ,, , and . Transition , and respectively represent
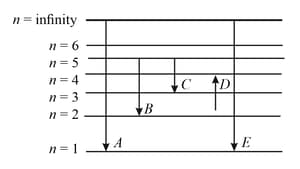
MEDIUM
JEE Main
IMPORTANT
In the given figure and respectively represent
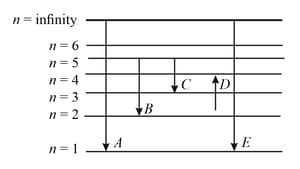 ,
,
MEDIUM
JEE Main
IMPORTANT
If the wavelength of the first line of the Balmer series of hydrogen is , the wavelength of the second line of the series should be?
EASY
JEE Main
IMPORTANT
The energy level diagram for a hydrogen-like atom is shown in the figure. The radius of its first Bohr orbit is

EASY
JEE Main
IMPORTANT
If the series limit of Lyman series for hydrogen atom is equal to the series limit of Balmer series for a hydrogen-like atom, then the atomic number of this hydrogen like atom will be
