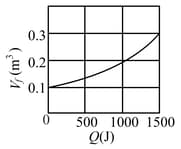
Suppose mole of an ideal gas undergoes an isothermal expansion as energy is added to it as heat . Graph shows the final volume versus . The temperature of the gas is :- (use and )


Important Questions on Thermodynamics
Reason: Adiabatic expansion of the gas causes lowering of temperature and condensation of water vapours.
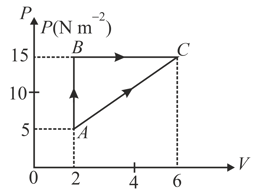
(i) The internal energy of gas at is and amount of heat supplied to change its state to through the path is . Calculate the internal energy at .
(ii) The internal energy of gas at is . Find the amount of heat supplied to the gas from to .
(i) If work done by the gas is then find the value of .
(ii) If increase in internal energy is then find the value of .
(iii) If amount of heat supplied is then find the value of .
(iv) If molar specific heat of the gas is then find the value of .
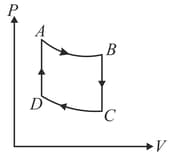
: Adiabatic expansion : Cooling at constant volume
: Adiabatic compression
: Heating at constant volume
The pressure and temperature at , etc., are denoted by etc., respectively. Given that , and .
Calculate the following quantities : (i) The work done by the gas in the process
(ii) The heat lost by the gas in the process
(iii) The temperature . (Given : )
At two moles of an ideal monoatomic gas occupy a volume . The gas expands adiabatically to a volume . Calculate:
(i) the final temperature of the gas,
(ii) change in its internal energy and
(iii) the work done by the gas during the process.
