MEDIUM
KVPY Aptitude Test - Stream SA
IMPORTANT
Earn 100
The density of a mixture of and at NTP is . Calculate the partial pressure of .

Important Questions on Gaseous State
MEDIUM
KVPY Aptitude Test - Stream SA
IMPORTANT
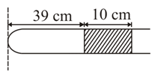
Given a one meter long glass tube closed at one end having a uniform cross-section containing a mercury column of length, at a distance of from the closed end. By what distance would this column move down, if the tube is held vertically with the open end downwards. [Take atmospheric pressure to be of ]
EASY
KVPY Aptitude Test - Stream SA
IMPORTANT
The plot that is not valid for an ideal gas where is the pressure and is the volume of the gas is:
EASY
KVPY Aptitude Test - Stream SA
IMPORTANT
One mole, each of the two gases and , are stored separately in two cylinders at at pressures and , respectively. The difference in the compressibilities of the two gases, , is:
EASY
KVPY Aptitude Test - Stream SA
IMPORTANT
An ideal gas is subjected to a cyclic change as shown in the diagram below:
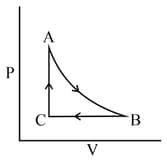
The step in which the gas will cool down is along:
EASY
KVPY Aptitude Test - Stream SA
IMPORTANT
For an ideal gas, Boyle's law is best described by -
EASY
KVPY Aptitude Test - Stream SA
IMPORTANT
The gas that has the slowest rate of diffusion among and is:
MEDIUM
KVPY Aptitude Test - Stream SA
IMPORTANT
Assuming ideal behaviour, the ratio of the kinetic energies of of and of at any temperature is:
MEDIUM
KVPY Aptitude Test - Stream SA
IMPORTANT
Consider two sealed jars of equal volume. One contains of hydrogen at and the other contains of nitrogen at . The gases in the two jars will have:
