HARD
JEE Main
IMPORTANT
Earn 100
The phase diagrams for a pure solvent (represented by the solid line) and a corresponding solution (containing a non-volatile solute and represented by the dashed lines) are shown below.
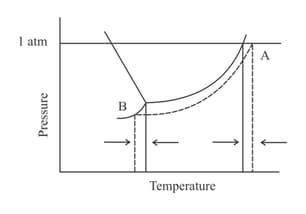
Choose the correct option.
Where and stand for freezing point temperature, boiling point temperature and molality.

(a) and
(b) and
(c) and
(d) and
50% studentsanswered this correctly

Important Questions on Solutions
MEDIUM
JEE Main
IMPORTANT
In the depression of freezing point experiment, it is found that
HARD
JEE Main
IMPORTANT
A solution of urea (mol mass ) boils at at atmospheric pressure. If and for water are and respectively, the above solution will not freeze at:
MEDIUM
JEE Main
IMPORTANT
Among the following, which statement is/are not correct when mercuric iodide is added to the aqueous solution of potassium iodide?
EASY
JEE Main
IMPORTANT
Which of the following is/are not linked to the lowering of vapor pressure by a non-volatile solute?
MEDIUM
JEE Main
IMPORTANT
of a substance is present in of solution showing the osmotic pressure of at . Calculate the molar mass of substance. What will be the osmotic pressure if temperature is raised to
MEDIUM
JEE Main
IMPORTANT
Calculate of a solution obtained by mixing of solution (mass/vol.) of urea (molar mass 60 ) and of solution (mass/vol.) of cane-sugar (molar at .
MEDIUM
JEE Main
IMPORTANT
How can you remove the hard calcium carbonate layer of the egg without damaging its semipermeable membrane? Can this egg be inserted into a bottle with a narrow neck without distorting its shape? Explain the process that is involved.
EASY
JEE Main
IMPORTANT
Why an unripe mango placed in a concentrated salt solution to prepare pickle shrivels?
