HARD
JEE Main
IMPORTANT
Earn 100
Which of the following mixtures on neutralization will show a same rise in temperature?
(a) of of
(b) of of
(c) of of
(d) of of
100% studentsanswered this correctly

Important Questions on Thermodynamics
HARD
JEE Main
IMPORTANT
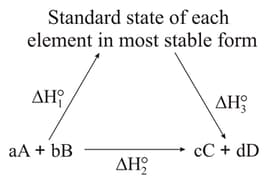
Which of the following are incorrect?
EASY
JEE Main
IMPORTANT
Which of the following reactions are exothermic?
MEDIUM
JEE Main
IMPORTANT
Select the correct statements.
HARD
JEE Main
IMPORTANT
Using the data (all values are in kilocalories per mole at ) given below, calculate the bond energy of and bonds.
combustion (ethane)
combustion (propane)
for (graphite)
Bond energy of
for
for
HARD
JEE Main
IMPORTANT
What is the bond energy if and ?
MEDIUM
JEE Main
IMPORTANT
Bond energies of and are and respectively. The heat liberated in the reaction is Find out the bond energy of .
MEDIUM
JEE Main
IMPORTANT
Calculate the resonance energy of from the following data:
of
MEDIUM
JEE Main
IMPORTANT
The enthalpy of combustion of at to give is and bond enthalpies of and are and , respectively. What will be the bond enthalpy of ?
