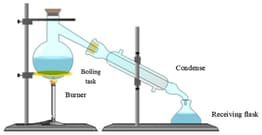
Distillation
Distillation: Overview
This topic covers concepts such as distillation for separation of two miscible liquids, distillation, fractional distillation, principle of fractional distillation, and setup for fractional distillation.
Important Questions on Distillation
Which of the following is an industrial application of fractional distillation?
The principle behind fractional distillation technique in separation of two liquids is:
A mixture of o-nitrophenol and p-nitrophenol can be separated by
A mixture of acetone and benzene can be separated by the following method
Which of the following techniques is used for separation of glycerol from soap in soap industry?
_____ is the process of separating the components or substances from a liquid mixture by using selective boiling and condensation.
Two volatile liquids A and B differ in their boiling points by . The process which can be used to separate them is
Name the method of separating the components of the following mixture: Sand and water
(Decantation / Distillation / Filtration)
Glycerol is separated from spent lye by :-
Name the method used to purify impure sample of benzene?
Label the figure given below.
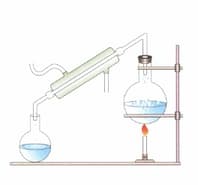
Where and why is it used?
Label the figure given below.
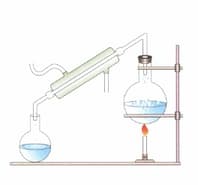
What is the process called?
State whether the following statements are True or False.
The process to obtain pure substance from a mixture in a solution is called distillation.
Observe the diagram carefully. Point out the place where water vapour condenses into water.
A liquid with boiling point189$ ℃$ is soluble in water.

Which of the following processes can be used to separate a colourless solution of the liquid from water?
State true or false.
The picture shows the formation of water vapour inside the condenser during the distillation of salt solution. These water vapours are free from any dissolved solid impurities.
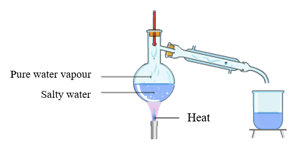
In a severe water contamination in home water supply which of the following is the recommended method for removal of soluble impurities from water?
Ink is soluble in water. How can you get pure water from a mixture of ink and water?
Which mixture can be separated using distillation?
Distillation is the process by which a pure substance is obtained from a mixture.