39 Insightful Publications
What is Soap?
Soap is a sodium or potassium salt of a long-chain fatty acid. It is made up of two parts: a long hydrocarbon part, called a hydrophobic tail, and a short ionic part, called a hydrophilic head.
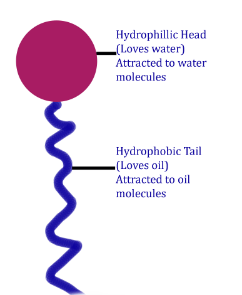
Hydrophilic and Hydrophobic Parts
The hydrophilic (water-attracting) part gets attracted towards water, while the hydrophobic (water-hating) carbon chain repels water and interacts with non-polar molecules such as oil, dirt, etc.
MICELLE: All the hydrophilic parts face towards the water, whereas all the hydrophobic parts face inwards, thus forming a cluster called a micelle.
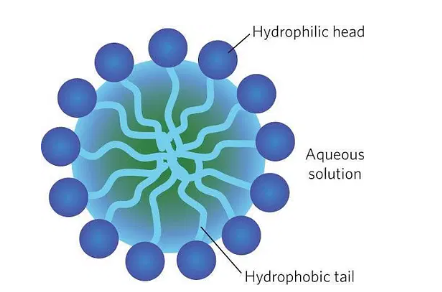
When soap is shaken with water, foam is produced due to the formation of micelle RCOO–Na+. Production of foam depends on the free availability and length of the hydrophobic portion of the soap. The foaming capacity of different soap samples can be compared by measuring the quantity of the foam produced by different soap samples.

This experiment will demonstrate the effect of soap in cleaning. Most dirt is oily in nature, and as you know, oil does not dissolve in water. The molecules of soap are sodium or potassium salts of long-chain carboxylic acids. The ionic end of soap interacts with water, while the carbon chain interacts with oil. The soap molecules, thus, form structures called micelles where one end of the molecules is towards the oil droplet while the ionic end faces outside. This forms an emulsion in water. The soap micelle thus helps in pulling out the dirt in water, and we can wash our clothes clean.
Let us understand this through a simulation, ‘Comparing the foaming capacity of soap samples’.
This experiment aims to compare the foaming capacity of various soap samples
The apparatus and chemicals required for this will be:
Apparatus:
- Test tubes
- Beakers
- Magnetic stirrers with stir bars
- Measuring ruler (15 cm)
- Watch glasses
- Droppers
- Corks
Chemicals:
- Distilled water
- Four different soap samples
The procedure followed for this experiment will be:
- Take four beakers labelled A, B, C & D containing 20 mL of distilled water.
- Dissolve 1 g of each of the four different soaps kept over the watch glass into beakers containing distilled water to prepare their respective soap solution.
- Pour 1 mL of each of the above-prepared soap solutions into corresponding test tubes.
- Add 5 mL of distilled water to each test tube.
- By shaking the test tube, the foam will be formed. Once the foam is formed, measure the length of the foam produced immediately to decide the foaming capacity of various soap samples.
Hence, it can be concluded that:
The length of the foam produced in each test tube containing the soap sample is measured using a measuring ruler to understand the foaming capacity of soap samples.
The soap sample that contained longer hydrophobic groups produced the maximum foaming capacity.
| Test Tube | Initial length (cm) | Final length (cm) | Length of the foam= Final length – Initial length (cm) |
|---|---|---|---|
| A | 4.5 | 7.0 | 2.5 |
| B | 4.5 | 5.5 | 1.0 |
| C | 4.5 | 5.0 | 0.5 |
| D | 4.5 | 4.8 | 0.3 |
The foaming capacity of different samples of soap was observed, and soap sample A had the greatest foaming capacity.
FAQs on Comparing the Foaming Capacity of Soap Samples
Q1. What are hydrocarbons?
Answer: Hydrocarbons are a class of compounds that contain carbon and hydrogen in them. They form the major component of petroleum products. Ex: Alkanes, Alkenes and Alkynes.
Q2. What do you mean by scum?
Answer: Scum is the precipitate formed when soap reacts with calcium and magnesium salts present in the water.
Q3. What do you understand about foaming capacity?
Answer: It is the capacity of the soap which makes more foam and that takes the longest to disappear.
Q4. Why does red litmus change its colour when dipped in a soap solution?
Answer: Soap is a salt of a strong base and a weak acid, and therefore, its pH is greater than 7. As a result, it shows action on red litmus.
Q5. Detergents form foam in hard water better than soaps because __________.
Answer: The salts formed by detergents with calcium and magnesium ions present in hard water are soluble. Hence, detergents form foam in hard water, whereas soap forms scum in hard water.




























