39 Insightful Publications
UNIT: CARBON AND ITS COMPOUNDS
Carbon and its compounds can be ACYCLIC Or OPEN-CHAIN COMPOUNDS (SATURATED HYDROCARBON AND UNSATURATED HYDROCARBONS) Or CYCLIC Or CLOSED CHAIN COMPOUNDS (Carbocyclic compounds Or Heterocyclic compounds).
Along with these compounds, in organic chemistry, some compounds contain a functional group, which is a specific group of atoms or bonds within a compound that is responsible for the characteristic chemical reactions of that compound.
For example:


Let us take an example of alcohols, carboxylic acid and esters and study esterification reaction.
Ethanoic acid is a colourless liquid. It’s boiling point is 391 K. It is miscible in water in all proportions.
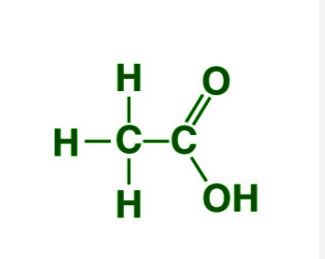
Ethanoic Acid
Ethanoic acid reacts with sodium. Due to this reaction, sodium ethanoate and hydrogen gas are formed.
2CH3COOH + 2Na → 2CH3COONa + H2
Ethanoic acid reacts with sodium hydroxide. Due to this reaction, sodium ethanoate and water are formed.
CH3COOH + NaOH → CH3COONa + H2O
Ethanoic acid reacts with sodium carbonate. Due to this reaction, sodium ethanoate, carbon dioxide and water are formed.
2CH3COOH + Na2CO3 → 2CH3COONa + CO2 + H2O
Reaction with alcohol
In the presence of a dilute acid or a base, ethanoic acid reacts with alcohols, to form esters, which have a fruity smell.
CH3COOH + C2H5OH ? CH3COOC2H5 + H2O
This process is known as esterification.
Esterification Reaction: Esters are most commonly formed by a reaction between a carboxylic acid and an alcohol in the presence of an acid catalyst such as concentrated sulphuric acid (H2SO4). Let’s take an example.
When ethanoic acid reacts with ethanol, the ethyl ethanoate ester is formed with the loss of a water molecule.
The reaction mixture comprising ethanoic acid, ethanol and a few drops of concentrated sulphuric acid is kept in a warm bath for some time. Later, the reaction mixture can be transferred to a solution containing sodium hydrogen carbonate to make sure that the unreacted ethanoic acid is removed from the mixture. After this, the formation of the ester can be confirmed by the smell coming out from the reaction mixture. The fruity, sweet smell is that of the ester. This reaction of ester formation is known as the esterification reaction.
The experiment aims to study the esterification reaction.
The apparatus and chemicals required for this experiment will be:
Apparatus:
- Test tube stand
- Test tube
- Dropper
- Glass rod
- Cork
- Measuring cylinder (10 mL)
- Beakers containing distilled water (250 mL): 2
- Burner
- Tripod Stand
- Wire gauze
.
Chemicals:
- Ethanoic acid: 3 mL
- Ethanol: 3 mL
- Conc. H2SO4
- Sodium hydrogen carbonate: 1 g
The procedure followed to study the esterification reaction will be:
- Take a test tube and clamp it to the stand.
- Measure 3 mL of ethanoic acid using a measuring cylinder and add it to the test tube.
- Measure 3 mL of ethanol in a measuring cylinder and add it to the test tube.
- Using a dropper, add
- Take a 250 mL beaker filled with distilled water. Keep it on a tripod stand with a wire gauze placed on it. Heat the beaker using a burner.
- Check the temperature of the water using a thermometer.
- Once the temperature reaches around 60 °C, place the test tube with the reaction mixture inside the beaker.
- As the reaction mixture gets warmed up slowly, shake it occasionally. Simultaneously take another 250 mL beaker and add 1 g of sodium hydrogen carbonate and 100 mL of water to it.
- After a few minutes, add the reaction mixture to the beaker containing the sodium hydrogen carbonate solution. This will remove the unreacted ethanoic acid from the reaction mixture.
- Try to recognise the smell of the vapours coming out.
Hence, it can be concluded that,
- The chemical reaction between ethanol (alcohol) and ethanoic acid (carboxylic acid) in the presence of conc. H2SO4
- The catalyst used for the esterification reaction is concentrated sulphuric acid.
- The products of the esterification reaction are ester and water.
- The unreacted acid in the ester mixture can be removed by reacting it with the solution of sodium hydrogen carbonate, which will neutralise the unreacted acid along with the formation of carbon dioxide and water.
NaHCO3 + H+ (unreacted acid) → CO2 + H2O + Salt of sodium
- An ester possesses a characteristic fruity smell.
FAQs on Esterification Reaction

Q1. What is the role of concentrated sulphuric acid in the esterification reaction?Conc. sulphuric acid helps in catalysing the esterification reaction.
ANSWER: A catalyst is a chemical entity that does not take part in a chemical reaction as a reactant or product, but helps the reaction to proceed faster.
Q2. Which gas is produced when ethanoic acid reacts with sodium hydrogen carbonate?
ANSWER: Ethanoic acid reacts with sodium hydrogen carbonate forming sodium ethanoate, water and carbon dioxide.
CH3COOH + NaHCO3 → CH3COONa + H2O + CO2
Q3. An ester is a class of organic compounds represented by the general formula R-COO-R’, where R and R’ represent the alkyl groups.
ANSWER: Esters are sweet smelling substances that are used as a constituent in perfumes and flavouring agents
Q4. Complete the given reaction and balance it.
2CH3COOH + 2Na ? ___ + H2
ANSWER: 2CH3COONa, Ethanoic acid reacts with sodium to form sodium ethanoate and hydrogen gas.
Q5. Name the substance other than conc. H2SO4 that can be used as a catalyst during the esterification reaction.
ANSWER: Acids other than conc. sulphuric acid, such as hydrochloric acid, can also be used as a catalyst for carrying out esterification reactions.




























