- Written By
Ankita Sahay
- Last Modified 15-02-2025
Dispersion Systems: Definition, Colloids, Types, Occurrence
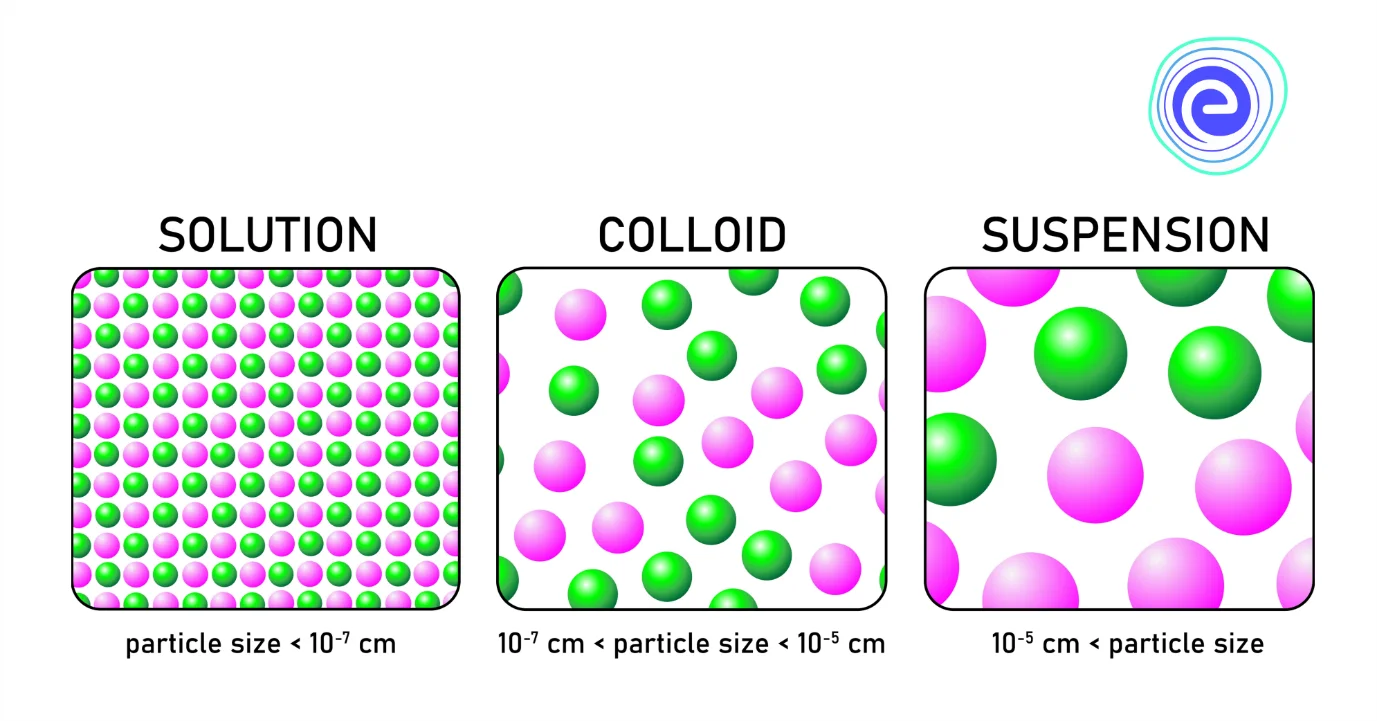
Dispersion System: Solutions are of two types, namely, homogeneous solutions and heterogeneous solutions. Suppose the composition of the solution is uniform throughout the solution. In that case, it is known as a homogeneous solution, and if the composition of the solution is non – uniform throughout the solution, it is known as a heterogeneous solution. They are of two types: suspensions and colloids. Suspensions are heterogeneous systems having particle sizes more than \(1000\,{\text{nm}}.\)
Colloids are heterogeneous systems with a particle size between \(1\,{\text{nm}}\) to \(1000\,{\text{nm}}.\) One substance is dispersed as very fine particles known as a dispersed phase in another substance called dispersion medium. A dispersion system is defined as a system in which distributed particles of one material or substance are dispersed in a continuous phase of another material. The two phases may be in similar or different states of matter. Dust in the air is the example of the dispersed phase, whereas the example of dispersion medium includes water in milk.
What is the Dispersed Phase?
The substance which is dispersed as very fine particles in a solution is called the dispersed phase. In a colloidal solution, a dispersed phase is suspended in the dispersion medium. A colloid solution does not possess a uniform mixture. It means that the particles of the dispersed phase are present in the particles of the dispersed medium in a colloidal solution.
Learn Everything About Sound Here
Examples of a heterogeneous mixture are water in starch or water in ink. Here water is dispersed medium, and starch or ink can be considered as a dispersed phase.
Such heterogeneous mixtures contain two phases that exist in this case. The stage that contains finely differentiated particles is known as the dispersed phase. A dispersed medium consists of two media that do not mix with each other; they can be in a liquid, a solid or a gas medium. Milk is made up of oil drops that are dispersed in water.
Classification of Dispersed Phase
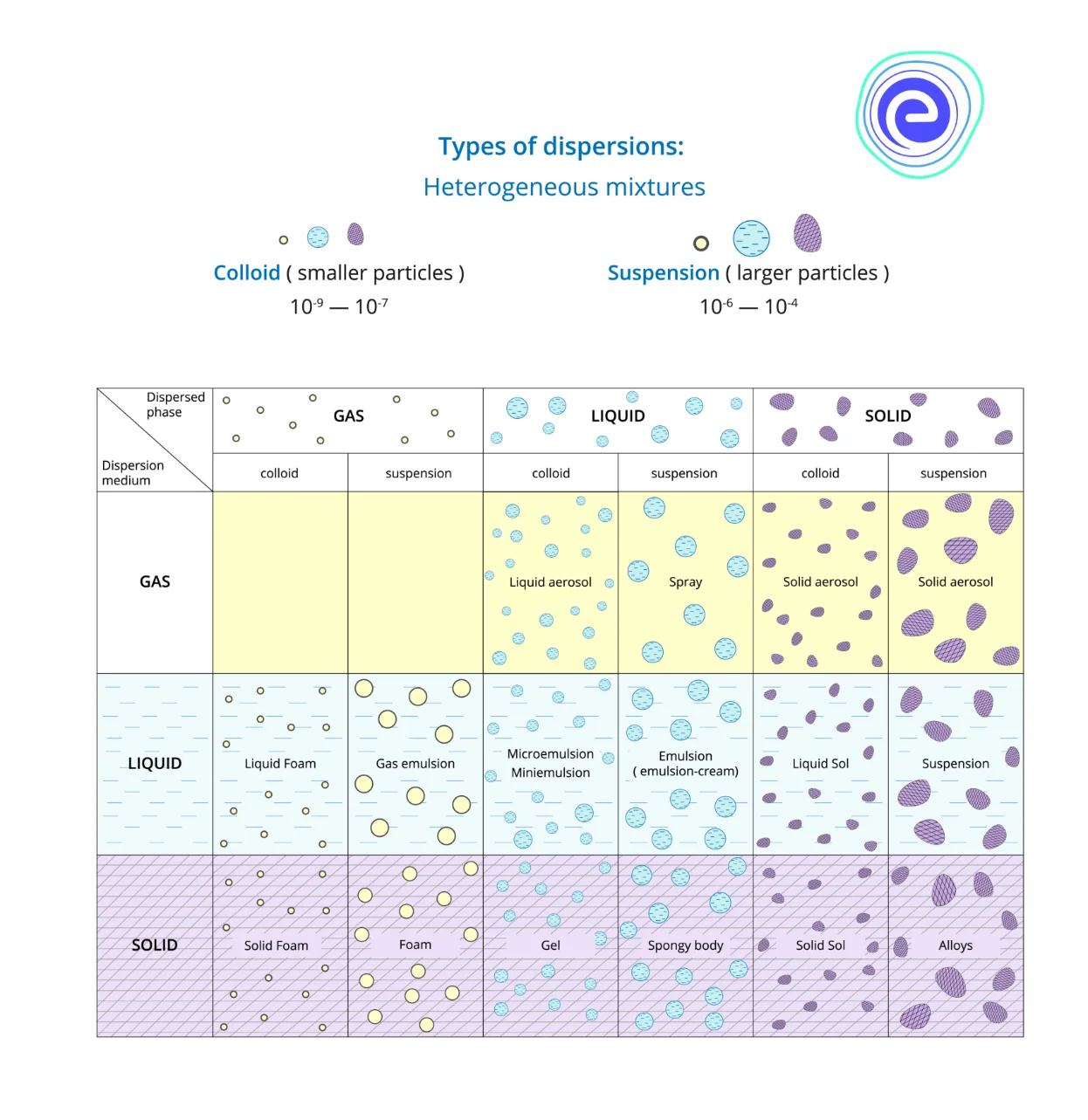
On the basis of the dispersed phase type, colloids are classified into various types that include sol, emulsion, foam and aerosol.
1. Sol – Sol is a colloidal suspension of solid particles in a liquid. For example, Ruby glass.
2. Emulsion – An emulsion is a colloidal suspension of two liquids like milk.
3. Foam – When gas particles are trapped in a solid or liquid, foam is formed, for example, soap in water.
4. Aerosols – When small particles of liquid or solid are dispersed in a gas, aerosols are formed—for example, smoke, fog, mist, etc.
Types of Colloids
Based on the nature of the minute particles of the dispersed phase, the colloids are mainly divided into three types:
1. Multimolecular colloids
2. Macromolecular Colloids
3. Associated Colloids
Let’s discuss each of them one by one:
1. Multimolecular Colloids: Usually, the size of such colloids varies from \(1 – 1000\,{\text{nm}}.\) When a substance is dissolved in a dispersion medium, it gets separated into many smaller molecules of variable sizes, and after the particles are separated, the colloid has several atoms and molecules, and such colloids are known as multimolecular colloids. For example, hundreds of sulphur molecules are held together by van der Waals force in the sulphur solution.
2. Macromolecular Colloids: As the name itself denotes, its size is relatively larger. However, in a suitable solvent, these macromolecular colloids form solutions whose size may or may not remain in the colloidal range. For example, cellulose, proteins, and enzymes are naturally formed macromolecular colloids, but rubber and polythene are synthesized macromolecular colloids.
3. Associated Colloids: Basically, these colloids behave like strong electrolytes. However, in higher concentrations, they show the behaviour of colloidal particles. Because of this nature, such colloids are known as Associated Colloids and popularly known as a micelle. For example, Micelle formation in soaps and detergents.
What is the Dispersed Medium?
The medium in which the colloidal particles are dispersed is known as the dispersion medium. It is the continuous phase of a colloid that gets distributed throughout the dispersion medium. Water in milk is an example of a dispersion medium.
What is Dispersion System?
The system in which the distributed particles of one material are dispersed in a continuous phase of another material is known as a dispersion system. The two phases may be either in the same or different states of matter. Clouds, fumes, clay, soil, etc., are some of the examples of dispersion systems.
Types of Dispersion Systems
Dispersion systems are of two types:
1. Molecular Dispersions – Molecular dispersions are referred to as solutions to a solute phase in the solvent. In this type of solution, the dispersed phase is homogeneously distributed in the dispersion medium. A common example of molecular dispersion is air which consists of various gasses like nitrogen, oxygen, carbon dioxide, water vapours, etc. Other examples of molecular dispersion are electrolytes and alloys.
2. Coarse Dispersions – Coarse dispersion is a heterogeneous dispersed system. Rapid sedimentation of the dispersed phase is observed in the coarse dispersions due to gravity.
Occurrence of Dispersion
The entire process of dispersion process is carried out by the two processes: molecular diffusion and convection. The coagulated particles get separated from each other, and a new line of interaction between the inner surface of the dispersion medium and the outer surface of dispersed particles is created. Through molecular diffusion phenomena, dispersion occurs in the entire bulk medium and in different concentrations. The difference between the concentration of the dispersed material and the bulk medium creates a concentration gradient that leads to the dispersion of the medium. In convection, variation in the velocity between the paths of the flow of particles in the bulk medium facilitates the equal distribution of dispersed material in the medium.
Downspout Dispersion Systems
1. The downspout is a pipe that carries rainwater water from the rooftop to the gutter. Thus, Downspout dispersion systems are trenches filled with splash blocks or gravel which serve to spread or carry roof runoff over the areas of vegetation.
2. Dispersion reduces the peak flow by slowing the runoff entering the conveyance system, allowing some infiltration, and improving the quality of water quality.
Summary of Dispersion Systems
In short, we can say that the solutions are of two types: homogeneous solutions and heterogeneous solutions. The homogeneous solution has a uniform composition throughout the solution, and the heterogeneous solution has non-uniform composition throughout the solution. Suspensions and colloids are the two types of heterogeneous solutions that depend upon particle size. Suspensions are heterogeneous systems having particle sizes more than \(1000\,{\text{nm}}.\) Colloids are heterogeneous systems with a particle size between \(1\,{\text{nm}}\) to \(1000\,{\text{nm}}.\) One substance is dispersed as very fine particles known as a dispersed phase in another substance called dispersion medium. The substance which is dispersed as very fine particles in a solution is called the dispersed phase.
In a colloidal solution, a dispersed phase is suspended in the dispersion medium. In a colloid solution, particles of the dispersed phase are present in the particles of the dispersed medium in a colloidal solution. Different types of dispersed phases are sol, emulsion, aerosol, and foam. Examples of a heterogeneous mixture are water in starch or water in ink. Here water is dispersed medium, and starch or ink can be considered as a dispersed phase. The system in which the distributed particles of one material are dispersed in a continuous phase of another material is known as a dispersion system. The two phases may be either in the same or different states of matter. Clouds, fumes, clay, soil, etc., are some of the examples of dispersion systems. Different types of dispersion systems are molecular dispersion and coarse dispersion.
FAQs on Dispersion Systems
Q.1. What are the different types of dispersion systems?
Ans: Dispersion systems are of two types:
(i) Molecular Dispersions – Molecular dispersions are referred to as solutions to a solute phase in the solvent. In this type of solution, the dispersed phase is homogeneously distributed in the dispersion medium. A common example of molecular dispersion is air which consists of various gasses like nitrogen, oxygen, carbon dioxide, water vapours, etc. Other examples of molecular dispersion are electrolytes and alloys.
(ii) Coarse Dispersions – The coarse dispersion is a heterogeneous dispersed system. Rapid sedimentation of the dispersed phase is observed in the coarse dispersions due to gravity.
Q.2. What are the typical characteristics of a dispersed system?
Ans: The system in which the distributed particles of one material are dispersed in a continuous phase of another material is known as a dispersion system. The two phases may be either in the same or different states of matter. Clouds, fumes, clay, soil, etc., are some of the examples of dispersion systems.
Q.3. What is the theory of dispersion?
Ans: Dispersion is the process by which the coagulated particles get separated from each other, and a new line of interaction between the inner surface of the dispersion medium and the outer surface of dispersed particles is created.
Q.4. What are the three types of dispersion?
Ans: Based on the pattern of the distribution of the particles in the solution, dispersion is of three types: Random dispersion, uniform dispersion, and clumped dispersion.
Q.5. What is the difference between the dispersed phase and dispersed medium?
Ans: Colloids are heterogeneous systems having a particle size between \(1000\,{\text{nm}}\) to \(1000\,{\text{nm}}\) in which one substance is dispersed as very fine particles known as a dispersed phase in another substance called dispersion medium. For example, Dust in the air is the example of the dispersed phase, whereas the example of dispersion medium includes water in milk.
Q.6. How does dispersion occur?
Ans: The entire process of dispersion process is carried out by the two processes: molecular diffusion and convection. The coagulated particles get separated from each other, and a new line of interaction between the inner surface of the dispersion medium and the outer surface of dispersed particles is created. Through molecular diffusion phenomena, dispersion occurs in the entire bulk medium and in different concentrations. The difference between the concentration of the dispersed material and the bulk medium creates a concentration gradient that leads to the dispersion of the medium. In convection, variation in the velocity between the paths of the flow of particles in the bulk medium facilitates the equal distribution of dispersed material in the medium.
Learn About Propagation of Sound Here
We hope this detailed article on Dispersion Systems is helpful to you. If you have any queries, drop a comment below, and we will get back to you.